Mechanisms of μ-opioid receptor inhibition of NMDA receptor-induced substance P release in the rat spinal cord
- PMID: 29042318
- PMCID: PMC5726399
- DOI: 10.1016/j.neuropharm.2017.10.014
Mechanisms of μ-opioid receptor inhibition of NMDA receptor-induced substance P release in the rat spinal cord
Abstract
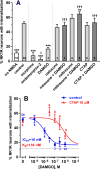
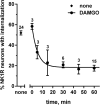
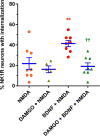
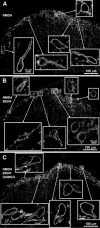
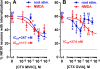
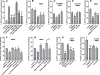
The interaction between NMDA receptors and μ-opioid receptors in primary afferent terminals was studied by using NMDA to induce substance P release, measured as neurokinin 1 receptor internalization. In rat spinal cord slices, the μ-opioid receptor agonists morphine, DAMGO and endomorphin-2 inhibited NMDA-induced substance P release, whereas the antagonist CTAP right-shifted the concentration response of DAMGO. In vivo, substance P release induced by intrathecal NMDA after priming with BDNF was inhibited by DAMGO. ω-Conotoxins MVIIC and GVIA inhibited about half of the NMDA-induced substance P release, showing that it was partially mediated by the opening of voltage-gated calcium (Cav) channels. In contrast, DAMGO or ω-conotoxins did not inhibit capsaicin-induced substance P release. In cultured DRG neurons, DAMGO but not ω-conotoxin inhibited NMDA-induced increases in intracellular calcium, indicating that μ-opioid receptors can inhibit NMDA receptor function by mechanisms other than inactivation of Cav channels. Moreover, DAMGO decreased the ω-conotoxin-insensitive component of the substance P release. Potent inhibition by ifenprodil showed that these NMDA receptors have the NR2B subunit. Activators of adenylyl cyclase and protein kinase A (PKA) induced substance P release and this was decreased by the NMDA receptor blocker MK-801 and by DAMGO. Conversely, inhibitors of adenylyl cyclase and PKA, but not of protein kinase C, decreased NMDA-induced substance P release. Hence, these NMDA receptors are positively modulated by the adenylyl cyclase-PKA pathway, which is inhibited by μ-opioid receptors. In conclusion, μ-opioid receptors inhibit NMDA receptor-induced substance P release through Cav channel inactivation and adenylyl cyclase inhibition.
Keywords: BVT948 (PubChem CID: 6604934); CTAP (PubChem CID: 90479802); Calcium channel; DAMGO (PubChem CID: 5462471); Endomorphin-2 (PubChem CID: 5311081); KT5720 (PubChem CID: 454202); Mu-opioid receptor; NMDA (PubChem CID: 22880); NMDA receptor; Neurokinin 1 receptor; Primary afferent; Protein kinase A; SQ22536 (PubChem CID: 5270); d-serine (PubChem CID: 71077); ω-conotoxin GVIA (PubChem CID: 73169082); ω-conotoxin MVIIC (PubChem CID: 56841670).
Published by Elsevier Ltd.
Figures


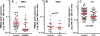

Similar articles
-
Neuropeptide Y release in the rat spinal cord measured with Y1 receptor internalization is increased after nerve injury.Neuropharmacology. 2019 Nov 1;158:107732. doi: 10.1016/j.neuropharm.2019.107732. Epub 2019 Aug 2. Neuropharmacology. 2019. PMID: 31377198 Free PMC article.
-
Mu-opioid and GABA(B) receptors modulate different types of Ca2+ currents in rat nodose ganglion neurons.Neuroscience. 1998 Aug;85(3):939-56. doi: 10.1016/s0306-4522(97)00674-x. Neuroscience. 1998. PMID: 9639286
-
NMDA receptors in primary afferents require phosphorylation by Src family kinases to induce substance P release in the rat spinal cord.Neuroscience. 2010 Mar 31;166(3):924-34. doi: 10.1016/j.neuroscience.2010.01.009. Epub 2010 Jan 13. Neuroscience. 2010. PMID: 20074620 Free PMC article.
-
Opioidergic control of the spinal release of neuropeptides. Possible significance for the analgesic effects of opioids.Fundam Clin Pharmacol. 1994;8(4):307-21. doi: 10.1111/j.1472-8206.1994.tb00809.x. Fundam Clin Pharmacol. 1994. PMID: 7851837 Review.
-
[Molecular neuropharmacology of nociceptive transmission and opioid receptors].Yakugaku Zasshi. 2000 Dec;120(12):1291-307. doi: 10.1248/yakushi1947.120.12_1291. Yakugaku Zasshi. 2000. PMID: 11193380 Review. Japanese.
Cited by
-
Dual mechanisms of opioid-induced respiratory depression in the inspiratory rhythm-generating network.Elife. 2021 Aug 17;10:e67523. doi: 10.7554/eLife.67523. Elife. 2021. PMID: 34402425 Free PMC article.
-
cAMP signaling through protein kinase A and Epac2 induces substance P release in the rat spinal cord.Neuropharmacology. 2021 May 15;189:108533. doi: 10.1016/j.neuropharm.2021.108533. Epub 2021 Mar 17. Neuropharmacology. 2021. PMID: 33744339 Free PMC article.
-
Chrysin ameliorates pain through regulation of the serum metabolomics in the rats.Korean J Pain. 2025 Apr 1;38(2):128-137. doi: 10.3344/kjp.24355. Epub 2025 Mar 20. Korean J Pain. 2025. PMID: 40107856 Free PMC article.
-
Neuropeptide Y release in the rat spinal cord measured with Y1 receptor internalization is increased after nerve injury.Neuropharmacology. 2019 Nov 1;158:107732. doi: 10.1016/j.neuropharm.2019.107732. Epub 2019 Aug 2. Neuropharmacology. 2019. PMID: 31377198 Free PMC article.
-
Mu-opioid receptors in nociceptive afferents produce a sustained suppression of hyperalgesia in chronic pain.Pain. 2018 Aug;159(8):1607-1620. doi: 10.1097/j.pain.0000000000001247. Pain. 2018. PMID: 29677019 Free PMC article.
References
-
- Aimone LD, Yaksh TL. Opioid modulation of capsaicin-evoked release of substance P from rat spinal cord in vivo. Peptides. 1989;10:1127–1131. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

