Antagonistic Transcription Factor Complexes Modulate the Floral Transition in Rice
- PMID: 29042404
- PMCID: PMC5728136
- DOI: 10.1105/tpc.17.00645
Antagonistic Transcription Factor Complexes Modulate the Floral Transition in Rice
Abstract
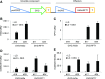
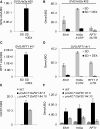
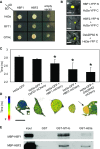
Plants measure day or night lengths to coordinate specific developmental changes with a favorable season. In rice (Oryza sativa), the reproductive phase is initiated by exposure to short days when expression of HEADING DATE 3a (Hd3a) and RICE FLOWERING LOCUS T 1 (RFT1) is induced in leaves. The cognate proteins are components of the florigenic signal and move systemically through the phloem to reach the shoot apical meristem (SAM). In the SAM, they form a transcriptional activation complex with the bZIP transcription factor OsFD1 to start panicle development. Here, we show that Hd3a and RFT1 can form transcriptional activation or repression complexes also in leaves and feed back to regulate their own transcription. Activation complexes depend on OsFD1 to promote flowering. However, additional bZIPs, including Hd3a BINDING REPRESSOR FACTOR1 (HBF1) and HBF2, form repressor complexes that reduce Hd3a and RFT1 expression to delay flowering. We propose that Hd3a and RFT1 are also active locally in leaves to fine-tune photoperiodic flowering responses.
© 2017 American Society of Plant Biologists. All rights reserved.
Figures




Similar articles
-
Functional diversification of FD transcription factors in rice, components of florigen activation complexes.Plant Cell Physiol. 2013 Mar;54(3):385-97. doi: 10.1093/pcp/pct005. Epub 2013 Jan 16. Plant Cell Physiol. 2013. PMID: 23324168 Free PMC article.
-
OsFD4 promotes the rice floral transition via florigen activation complex formation in the shoot apical meristem.New Phytol. 2021 Jan;229(1):429-443. doi: 10.1111/nph.16834. Epub 2020 Aug 27. New Phytol. 2021. PMID: 32737885
-
Hd3a promotes lateral branching in rice.Plant J. 2015 Apr;82(2):256-66. doi: 10.1111/tpj.12811. Plant J. 2015. PMID: 25740115
-
The coincidence of critical day length recognition for florigen gene expression and floral transition under long-day conditions in rice.Mol Plant. 2013 May;6(3):635-49. doi: 10.1093/mp/sst022. Epub 2013 Feb 15. Mol Plant. 2013. PMID: 23416454 Review.
-
Emerging insights into florigen transport.Curr Opin Plant Biol. 2013 Oct;16(5):607-13. doi: 10.1016/j.pbi.2013.06.001. Epub 2013 Jun 28. Curr Opin Plant Biol. 2013. PMID: 23810436 Review.
Cited by
-
Overexpression of RICE FLOWERING LOCUS T 1 (RFT1) Induces Extremely Early Flowering in Rice.Mol Cells. 2019 May 31;42(5):406-417. doi: 10.14348/molcells.2019.0009. Mol Cells. 2019. PMID: 31085810 Free PMC article.
-
Physiological factors influencing climate-smart agriculture: Daylength-mediated interaction between tillering and flowering in rice.BMC Plant Biol. 2025 Mar 31;25(1):400. doi: 10.1186/s12870-025-06430-z. BMC Plant Biol. 2025. PMID: 40159470 Free PMC article.
-
Functional divergence between soybean FLOWERING LOCUS T orthologues FT2a and FT5a in post-flowering stem growth.J Exp Bot. 2019 Aug 7;70(15):3941-3953. doi: 10.1093/jxb/erz199. J Exp Bot. 2019. PMID: 31035293 Free PMC article.
-
Environmental control of rice flowering time.Plant Commun. 2023 Sep 11;4(5):100610. doi: 10.1016/j.xplc.2023.100610. Epub 2023 May 4. Plant Commun. 2023. PMID: 37147799 Free PMC article. Review.
-
Contributions of Adaptive Plant Architecture to Transgressive Salinity Tolerance in Recombinant Inbred Lines of Rice: Molecular Mechanisms Based on Transcriptional Networks.Front Genet. 2020 Oct 23;11:594569. doi: 10.3389/fgene.2020.594569. eCollection 2020. Front Genet. 2020. PMID: 33193743 Free PMC article.
References
-
- Abelenda J.A., Cruz-Oró E., Franco-Zorrilla J.M., Prat S. (2016). Potato StCONSTANS-like1 suppresses storage organ formation by directly activating the FT-like StSP5G repressor. Curr. Biol. 26: 872–881. - PubMed
-
- Andrés F., Coupland G. (2012). The genetic basis of flowering responses to seasonal cues. Nat. Rev. Genet. 13: 627–639. - PubMed
-
- Brambilla V., Fornara F. (2013). Molecular control of flowering in response to day length in rice. J. Integr. Plant Biol. 55: 410–418. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

