Environmental and Genetic Factors Regulating Localization of the Plant Plasma Membrane H+-ATPase
- PMID: 29042459
- PMCID: PMC5761788
- DOI: 10.1104/pp.17.01126
Environmental and Genetic Factors Regulating Localization of the Plant Plasma Membrane H+-ATPase
Abstract
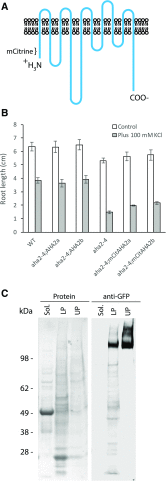
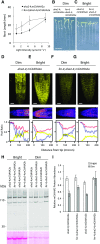
A P-type H+-ATPase is the primary transporter that converts ATP to electrochemical energy at the plasma membrane of higher plants. Its product, the proton-motive force, is composed of an electrical potential and a pH gradient. Many studies have demonstrated that this proton-motive force not only drives the secondary transporters required for nutrient uptake, but also plays a direct role in regulating cell expansion. Here, we have generated a transgenic Arabidopsis (Arabidopsis thaliana) plant expressing H+-ATPase isoform 2 (AHA2) that is translationally fused with a fluorescent protein and examined its cellular localization by live-cell microscopy. Using a 3D imaging approach with seedlings grown for various times under a variety of light intensities, we demonstrate that AHA2 localization at the plasma membrane of root cells requires light. In dim light conditions, AHA2 is found in intracellular compartments, in addition to the plasma membrane. This localization profile was age-dependent and specific to cell types found in the transition zone located between the meristem and elongation zones. The accumulation of AHA2 in intracellular compartments is consistent with reduced H+ secretion near the transition zone and the suppression of root growth. By examining AHA2 localization in a knockout mutant of a receptor protein kinase, FERONIA, we found that the intracellular accumulation of AHA2 in the transition zone is dependent on a functional FERONIA-dependent inhibitory response in root elongation. Overall, this study provides a molecular underpinning for understanding the genetic, environmental, and developmental factors influencing root growth via localization of the plasma membrane H+-ATPase.
© 2018 American Society of Plant Biologists. All Rights Reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

