Differential Response of Glioma Stem Cells to Arsenic Trioxide Therapy Is Regulated by MNK1 and mRNA Translation
- PMID: 29042487
- PMCID: PMC5926791
- DOI: 10.1158/1541-7786.MCR-17-0397
Differential Response of Glioma Stem Cells to Arsenic Trioxide Therapy Is Regulated by MNK1 and mRNA Translation
Abstract
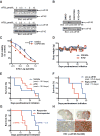
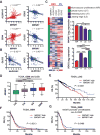
Mesenchymal (MES) and proneural (PN) are two distinct glioma stem cell (GSC) populations that drive therapeutic resistance in glioblastoma (GBM). We screened a panel of 650 small molecules against patient-derived GBM cells to discover compounds targeting specific GBM subtypes. Arsenic trioxide (ATO), an FDA-approved drug that crosses the blood-brain barrier, was identified as a potent PN-specific compound in the initial screen and follow-up validation studies. Furthermore, MES and PN GSCs exhibited differential sensitivity to ATO. As ATO has been shown to activate the MAPK-interacting kinase 1 (MNK1)-eukaryotic translation initiation factor 4E (eIF4E) pathway and subsequent mRNA translation in a negative regulatory feedback manner, the mechanistic role of ATO resistance in MES GBM was explored. In GBM cells, ATO-activated translation initiation cellular events via the MNK1-eIF4E signaling axis. Furthermore, resistance to ATO in intracranial PDX tumors correlated with high eIF4E phosphorylation. Polysomal fractionation and microarray analysis of GBM cells were performed to identify ATO's effect on mRNA translation and enrichment of anti-apoptotic mRNAs in the ATO-induced translatome was found. Additionally, it was determined that MNK inhibition sensitized MES GSCs to ATO in neurosphere and apoptosis assays. Finally, examination of the effect of ATO on patients from a phase I/II clinical trial of ATO revealed that PN GBM patients responded better to ATO than other subtypes as demonstrated by longer overall and progression-free survival.Implications: These findings raise the possibility of a unique therapeutic approach for GBM, involving MNK1 targeting to sensitize MES GSCs to drugs like arsenic trioxide. Mol Cancer Res; 16(1); 32-46. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
P. Kumthekar is a medical advisory board member for Vivacitas; and is a consultant/advisory board member at Abbvie. J. Raizer is an Executive Medical Director at Celldex; reports receiving commercial research grants from Astra-Zeneca and Genentech; and is a consultant/advisory board member for Zio-Pharm. No potential conflicts of interest were disclosed by the other authors.
Figures





Similar articles
-
MNK Inhibition Disrupts Mesenchymal Glioma Stem Cells and Prolongs Survival in a Mouse Model of Glioblastoma.Mol Cancer Res. 2016 Oct;14(10):984-993. doi: 10.1158/1541-7786.MCR-16-0172. Epub 2016 Jun 30. Mol Cancer Res. 2016. PMID: 27364770 Free PMC article.
-
MAP kinase-interacting kinase 1 regulates SMAD2-dependent TGF-β signaling pathway in human glioblastoma.Cancer Res. 2011 Mar 15;71(6):2392-402. doi: 10.1158/0008-5472.CAN-10-3112. Epub 2011 Mar 14. Cancer Res. 2011. PMID: 21406405
-
Inhibition of MNK pathways enhances cancer cell response to chemotherapy with temozolomide and targeted radionuclide therapy.Cell Signal. 2016 Sep;28(9):1412-1421. doi: 10.1016/j.cellsig.2016.06.005. Epub 2016 Jun 8. Cell Signal. 2016. PMID: 27289018
-
Stem cell signature in glioblastoma: therapeutic development for a moving target.J Neurosurg. 2015 Feb;122(2):324-30. doi: 10.3171/2014.9.JNS132253. Epub 2014 Nov 14. J Neurosurg. 2015. PMID: 25397368 Review.
-
Mitogen-activated Protein Kinase (MAPK) Interacting Kinases 1 and 2 (MNK1 and MNK2) as Targets for Cancer Therapy: Recent Progress in the Development of MNK Inhibitors.Curr Med Chem. 2017;24(28):3025-3053. doi: 10.2174/0929867324666170203123427. Curr Med Chem. 2017. PMID: 28164761 Review.
Cited by
-
Identification and targeting of novel CDK9 complexes in acute myeloid leukemia.Blood. 2019 Mar 14;133(11):1171-1185. doi: 10.1182/blood-2018-08-870089. Epub 2018 Dec 26. Blood. 2019. PMID: 30587525 Free PMC article.
-
MAP Kinase-Interacting Kinase 1 Promotes Proliferation and Invasion of Hepatocellular Carcinoma and Is an Unfavorable Prognostic Biomarker.Med Sci Monit. 2018 Mar 26;24:1759-1767. doi: 10.12659/msm.909012. Med Sci Monit. 2018. PMID: 29576605 Free PMC article.
-
PRP‑1 significantly decreases the ALDHhigh cancer stem cell population and regulates the aberrant Wnt/β‑catenin pathway in human chondrosarcoma JJ012 cells.Oncol Rep. 2019 Jul;42(1):103-114. doi: 10.3892/or.2019.7172. Epub 2019 May 24. Oncol Rep. 2019. PMID: 31180539 Free PMC article.
-
Evaluating the Efficacy of Siddha Pharmacotherapy for Acrochordon: A Case Report.Cureus. 2024 Dec 25;16(12):e76391. doi: 10.7759/cureus.76391. eCollection 2024 Dec. Cureus. 2024. PMID: 39872594 Free PMC article.
-
Potent Antineoplastic Effects of Combined PI3Kα-MNK Inhibition in Medulloblastoma.Mol Cancer Res. 2019 Jun;17(6):1305-1315. doi: 10.1158/1541-7786.MCR-18-1193. Epub 2019 Mar 6. Mol Cancer Res. 2019. PMID: 30842251 Free PMC article.
References
-
- Nakano I. Stem cell signature in glioblastoma: therapeutic development for a moving target. J Neurosurg. 2015;122:324–30. - PubMed
-
- Segerman A, Niklasson M, Haglund C, Bergstrom T, Jarvius M, Xie Y, et al. Clonal variation in drug and radiation response among glioma-initiating cells is linked to proneural-mesenchymal transition. Cell Rep. 2016;17:2994–3009. - PubMed
-
- Au WY, Tam S, Fong BM, Kwong YL. Determinants of cerebrospinal fluid arsenic concentration in patients with acute promyelocytic leukemia on oral arsenic trioxide therapy. Blood. 2008;112:3587–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA121192/CA/NCI NIH HHS/United States
- R01 CA077816/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- I01 CX000916/CX/CSRD VA/United States
- U01 CA168397/CA/NCI NIH HHS/United States
- R01 CA159811/CA/NCI NIH HHS/United States
- R01 NS093843/NS/NINDS NIH HHS/United States
- T32 CA070085/CA/NCI NIH HHS/United States
- T32 GM008152/GM/NIGMS NIH HHS/United States
- T32 GM144295/GM/NIGMS NIH HHS/United States
- R01 NS095634/NS/NINDS NIH HHS/United States
- R21 CA209345/CA/NCI NIH HHS/United States
- R01 CA155566/CA/NCI NIH HHS/United States
- P30 CA030199/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

