A Single Dual-Function Enzyme Controls the Production of Inflammatory NOD Agonist Peptidoglycan Fragments by Neisseria gonorrhoeae
- PMID: 29042497
- PMCID: PMC5646250
- DOI: 10.1128/mBio.01464-17
A Single Dual-Function Enzyme Controls the Production of Inflammatory NOD Agonist Peptidoglycan Fragments by Neisseria gonorrhoeae
Abstract
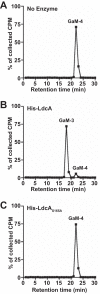
Neisseria gonorrhoeae gonococcus (GC) is a Gram-negative betaproteobacterium and causative agent of the sexually transmitted infection gonorrhea. During growth, GC releases lipooligosaccharide (LOS) and peptidoglycan (PG) fragments, which contribute significantly to the inflammatory damage observed during human infection. In ascending infection of human Fallopian tubes, inflammation leads to increased risk of ectopic pregnancy, pelvic inflammatory disease, and sterility. Of the PG fragments released by GC, most are disaccharide peptide monomers, and of those, 80% have tripeptide stems despite the observation that tetrapeptide stems make up 80% of the assembled cell wall. We identified a serine-protease l,d-carboxypeptidase, NGO1274 (LdcA), as the enzyme responsible for converting cell wall tetrapeptide-stem PG to released tripeptide-stem PG. Unlike characterized cytoplasmic LdcA homologs in gammaproteobacteria, LdcA in GC is exported to the periplasm, and its localization is critical for its activity in modifying PG fragments for release. Distinct among other characterized l,d-carboxypeptidases, LdcA from GC is also capable of catalyzing the cleavage of specific peptide cross-bridges (endopeptidase activity). To define the role of ldcA in pathogenesis, we demonstrate that ldcA disruption results in both loss of NOD1-dependent NF-κB activation and decreased NOD2-dependent NF-κB activation while not affecting Toll-like receptor (TLR) agonist release. Since the human intracellular peptidoglycan receptor NOD1 (hNOD1) specifically recognizes PG fragments with a terminal meso-DAP rather than d-alanine, we conclude that LdcA is required for GC to provoke NOD1-dependent responses in cells of the human host.IMPORTANCE The macromolecular meshwork of peptidoglycan serves essential functions in determining bacterial cell shape, protecting against osmotic lysis, and defending cells from external assaults. The conserved peptidoglycan structure, however, is also recognized by eukaryotic pattern recognition receptors, which can trigger immune responses against bacteria. Many bacteria can induce an inflammatory response through the intracellular peptidoglycan receptor NOD1, but Neisseria gonorrhoeae serves as an extreme example, releasing fragments of peptidoglycan into the environment during growth that specifically antagonize human NOD1. Understanding the peptidoglycan breakdown mechanisms that allow Neisseria to promote NOD1 activation, rather than avoiding or suppressing immune detection, is critical to understanding the pathogenesis of this increasingly drug-resistant organism. We identify a peptidoglycan l,d-carboxypeptidase responsible for converting liberated peptidoglycan fragments into the human NOD1 agonist and find that the same enzyme has endopeptidase activity on certain peptidoglycan cross-links, the first described combination of those two activities in a single enzyme.
Keywords: Neisseria gonorrhoeae; high-pressure liquid chromatography; innate immunity; peptidoglycan; peptidoglycan hydrolases.
Copyright © 2017 Lenz et al.
Figures


Similar articles
-
Peptidoglycan fragment release and NOD activation by commensal Neisseria species from humans and other animals.Infect Immun. 2024 May 7;92(5):e0000424. doi: 10.1128/iai.00004-24. Epub 2024 Apr 2. Infect Immun. 2024. PMID: 38563734 Free PMC article.
-
Neisseria gonorrhoeae PBP3 and PBP4 Facilitate NOD1 Agonist Peptidoglycan Fragment Release and Survival in Stationary Phase.Infect Immun. 2019 Jan 24;87(2):e00833-18. doi: 10.1128/IAI.00833-18. Print 2019 Feb. Infect Immun. 2019. PMID: 30510100 Free PMC article.
-
Activation of NOD receptors by Neisseria gonorrhoeae modulates the innate immune response.Innate Immun. 2014 May;20(4):377-89. doi: 10.1177/1753425913493453. Epub 2013 Jul 24. Innate Immun. 2014. PMID: 23884094 Free PMC article.
-
The Pathogenic Neisseria Use a Streamlined Set of Peptidoglycan Degradation Proteins for Peptidoglycan Remodeling, Recycling, and Toxic Fragment Release.Front Microbiol. 2019 Jan 31;10:73. doi: 10.3389/fmicb.2019.00073. eCollection 2019. Front Microbiol. 2019. PMID: 30766523 Free PMC article. Review.
-
Attention Seeker: Production, Modification, and Release of Inflammatory Peptidoglycan Fragments in Neisseria Species.J Bacteriol. 2017 Sep 19;199(20):e00354-17. doi: 10.1128/JB.00354-17. Print 2017 Oct 15. J Bacteriol. 2017. PMID: 28674065 Free PMC article. Review.
Cited by
-
Mutation of mltG increases peptidoglycan fragment release, cell size, and antibiotic susceptibility in Neisseria gonorrhoeae.J Bacteriol. 2023 Dec 19;205(12):e0027723. doi: 10.1128/jb.00277-23. Epub 2023 Dec 1. J Bacteriol. 2023. PMID: 38038461 Free PMC article.
-
Interplay between Peptidoglycan Biology and Virulence in Gram-Negative Pathogens.Microbiol Mol Biol Rev. 2018 Sep 12;82(4):e00033-18. doi: 10.1128/MMBR.00033-18. Print 2018 Dec. Microbiol Mol Biol Rev. 2018. PMID: 30209071 Free PMC article. Review.
-
Efficacy and Mechanism of the Action of Live and Heat-Killed Bacillus coagulans BC198 as Potential Probiotic in Ameliorating Dextran Sulfate Sodium-Induced Colitis in Mice.ACS Omega. 2024 Feb 20;9(9):10253-10266. doi: 10.1021/acsomega.3c07529. eCollection 2024 Mar 5. ACS Omega. 2024. PMID: 38463297 Free PMC article.
-
Role of endopeptidases in peptidoglycan synthesis mediated by alternative cross-linking enzymes in Escherichia coli.EMBO J. 2021 Oct 1;40(19):e108126. doi: 10.15252/embj.2021108126. Epub 2021 Aug 12. EMBO J. 2021. PMID: 34382698 Free PMC article.
-
Pathogenesis of Neisseria gonorrhoeae and the Host Defense in Ascending Infections of Human Fallopian Tube.Front Immunol. 2018 Nov 21;9:2710. doi: 10.3389/fimmu.2018.02710. eCollection 2018. Front Immunol. 2018. PMID: 30524442 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

