Unintended Laboratory-Driven Evolution Reveals Genetic Requirements for Biofilm Formation by Desulfovibrio vulgaris Hildenborough
- PMID: 29042504
- PMCID: PMC5646257
- DOI: 10.1128/mBio.01696-17
Unintended Laboratory-Driven Evolution Reveals Genetic Requirements for Biofilm Formation by Desulfovibrio vulgaris Hildenborough
Abstract
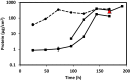
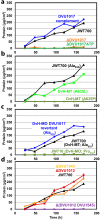
Biofilms of sulfate-reducing bacteria (SRB) are of particular interest as members of this group are culprits in corrosion of industrial metal and concrete pipelines as well as being key players in subsurface metal cycling. Yet the mechanism of biofilm formation by these bacteria has not been determined. Here we show that two supposedly identical wild-type cultures of the SRB Desulfovibrio vulgaris Hildenborough maintained in different laboratories have diverged in biofilm formation. From genome resequencing and subsequent mutant analyses, we discovered that a single nucleotide change within DVU1017, the ABC transporter of a type I secretion system (T1SS), was sufficient to eliminate biofilm formation in D. vulgaris Hildenborough. Two T1SS cargo proteins were identified as likely biofilm structural proteins, and the presence of at least one (with either being sufficient) was shown to be required for biofilm formation. Antibodies specific to these biofilm structural proteins confirmed that DVU1017, and thus the T1SS, is essential for localization of these adhesion proteins on the cell surface. We propose that DVU1017 is a member of the lapB category of microbial surface proteins because of its phenotypic similarity to the adhesin export system described for biofilm formation in the environmental pseudomonads. These findings have led to the identification of two functions required for biofilm formation in D. vulgaris Hildenborough and focus attention on the importance of monitoring laboratory-driven evolution, as phenotypes as fundamental as biofilm formation can be altered.IMPORTANCE The growth of bacteria attached to a surface (i.e., biofilm), specifically biofilms of sulfate-reducing bacteria, has a profound impact on the economy of developed nations due to steel and concrete corrosion in industrial pipelines and processing facilities. Furthermore, the presence of sulfate-reducing bacteria in oil wells causes oil souring from sulfide production, resulting in product loss, a health hazard to workers, and ultimately abandonment of wells. Identification of the required genes is a critical step for determining the mechanism of biofilm formation by sulfate reducers. Here, the transporter by which putative biofilm structural proteins are exported from sulfate-reducing Desulfovibrio vulgaris Hildenborough cells was discovered, and a single nucleotide change within the gene coding for this transporter was found to be sufficient to completely stop formation of biofilm.
Keywords: Desulfovibrio vulgaris; biofilms; genetic polymorphisms; secretion systems; sulfate reduction.
Copyright © 2017 De León et al.
Figures




Similar articles
-
Absence of biofilm adhesin proteins changes surface attachment and cell strategy for Desulfovibrio vulgaris Hildenborough.J Bacteriol. 2025 Jan 31;207(1):e0037924. doi: 10.1128/jb.00379-24. Epub 2024 Dec 31. J Bacteriol. 2025. PMID: 39745371 Free PMC article.
-
Effect of Quorum Sensing on the Ability of Desulfovibrio vulgaris To Form Biofilms and To Biocorrode Carbon Steel in Saline Conditions.Appl Environ Microbiol. 2019 Dec 13;86(1):e01664-19. doi: 10.1128/AEM.01664-19. Print 2019 Dec 13. Appl Environ Microbiol. 2019. PMID: 31628147 Free PMC article.
-
Transcriptomic and proteomic analyses of Desulfovibrio vulgaris biofilms: carbon and energy flow contribute to the distinct biofilm growth state.BMC Genomics. 2012 Apr 16;13:138. doi: 10.1186/1471-2164-13-138. BMC Genomics. 2012. PMID: 22507456 Free PMC article.
-
The adaptive genome of Desulfovibrio vulgaris Hildenborough.FEMS Microbiol Lett. 2006 Jul;260(2):127-33. doi: 10.1111/j.1574-6968.2006.00261.x. FEMS Microbiol Lett. 2006. PMID: 16842335 Review.
-
Desulfovibrio vulgaris as a model microbe for the study of corrosion under sulfate-reducing conditions.mLife. 2022 Mar 24;1(1):13-20. doi: 10.1002/mlf2.12018. eCollection 2022 Mar. mLife. 2022. PMID: 38818327 Free PMC article. Review.
Cited by
-
H2 Is a Major Intermediate in Desulfovibrio vulgaris Corrosion of Iron.mBio. 2023 Apr 25;14(2):e0007623. doi: 10.1128/mbio.00076-23. Epub 2023 Feb 14. mBio. 2023. PMID: 36786581 Free PMC article.
-
Reconstitution of a biofilm adhesin system from a sulfate-reducing bacterium in Pseudomonas fluorescens.Proc Natl Acad Sci U S A. 2024 Mar 26;121(13):e2320410121. doi: 10.1073/pnas.2320410121. Epub 2024 Mar 18. Proc Natl Acad Sci U S A. 2024. PMID: 38498718 Free PMC article.
-
Reconstitution of a Biofilm Adhesin System from a Sulfate-Reducing Bacterium in Pseudomonas fluorescens.bioRxiv [Preprint]. 2023 Nov 22:2023.11.22.568322. doi: 10.1101/2023.11.22.568322. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2024 Mar 26;121(13):e2320410121. doi: 10.1073/pnas.2320410121. PMID: 38045380 Free PMC article. Updated. Preprint.
-
Deletion Mutants, Archived Transposon Library, and Tagged Protein Constructs of the Model Sulfate-Reducing Bacterium Desulfovibrio vulgaris Hildenborough.Microbiol Resour Announc. 2021 Mar 18;10(11):e00072-21. doi: 10.1128/MRA.00072-21. Microbiol Resour Announc. 2021. PMID: 33737356 Free PMC article.
-
An N-Terminal Retention Module Anchors the Giant Adhesin LapA of Pseudomonas fluorescens at the Cell Surface: a Novel Subfamily of Type I Secretion Systems.J Bacteriol. 2018 Mar 26;200(8):e00734-17. doi: 10.1128/JB.00734-17. Print 2018 Apr 15. J Bacteriol. 2018. PMID: 29437852 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
