The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases
- PMID: 29042534
- PMCID: PMC5645390
- DOI: 10.1038/s41467-017-00901-0
The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases
Abstract
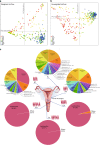
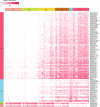
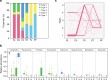
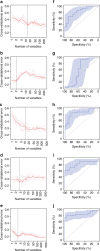
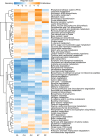
Reports on bacteria detected in maternal fluids during pregnancy are typically associated with adverse consequences, and whether the female reproductive tract harbours distinct microbial communities beyond the vagina has been a matter of debate. Here we systematically sample the microbiota within the female reproductive tract in 110 women of reproductive age, and examine the nature of colonisation by 16S rRNA gene amplicon sequencing and cultivation. We find distinct microbial communities in cervical canal, uterus, fallopian tubes and peritoneal fluid, differing from that of the vagina. The results reflect a microbiota continuum along the female reproductive tract, indicative of a non-sterile environment. We also identify microbial taxa and potential functions that correlate with the menstrual cycle or are over-represented in subjects with adenomyosis or infertility due to endometriosis. The study provides insight into the nature of the vagino-uterine microbiome, and suggests that surveying the vaginal or cervical microbiota might be useful for detection of common diseases in the upper reproductive tract.Whether the female reproductive tract harbours distinct microbiomes beyond the vagina has been a matter of debate. Here, the authors show a subject-specific continuity in microbial communities at six sites along the female reproductive tract, indicative of a non-sterile environment.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

