Mechanocaloric effects in superionic thin films from atomistic simulations
- PMID: 29042557
- PMCID: PMC5645463
- DOI: 10.1038/s41467-017-01081-7
Mechanocaloric effects in superionic thin films from atomistic simulations
Abstract
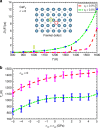
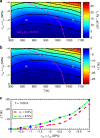
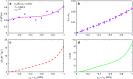
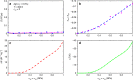
Solid-state cooling is an energy-efficient and scalable refrigeration technology that exploits the adiabatic variation of a crystalline order parameter under an external field (electric, magnetic, or mechanic). The mechanocaloric effect bears one of the greatest cooling potentials in terms of energy efficiency owing to its large available latent heat. Here we show that giant mechanocaloric effects occur in thin films of well-known families of fast-ion conductors, namely Li-rich (Li3OCl) and type-I (AgI), an abundant class of materials that routinely are employed in electrochemistry cells. Our simulations reveal that at room temperature AgI undergoes an adiabatic temperature shift of 38 K under a biaxial stress of 1 GPa. Likewise, Li3OCl displays a cooling capacity of 9 K under similar mechanical conditions although at a considerably higher temperature. We also show that ionic vacancies have a detrimental effect on the cooling performance of superionic thin films. Our findings should motivate experimental mechanocaloric searches in a wide variety of already known superionic materials.Mechanocaloric effects are a promising path towards solid-state cooling. Here the authors perform atomistic simulations on the well-known fast-ion conductor silver iodide and computationally predict a sizeable mechanocaloric effect under biaxial strain.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Room-temperature mechanocaloric effects in lithium-based superionic materials.Nat Commun. 2018 Aug 20;9(1):3337. doi: 10.1038/s41467-018-05835-9. Nat Commun. 2018. PMID: 30127398 Free PMC article.
-
Giant Mechanocaloric Effects in Fluorite-Structured Superionic Materials.Nano Lett. 2016 May 11;16(5):3124-9. doi: 10.1021/acs.nanolett.6b00422. Epub 2016 Apr 14. Nano Lett. 2016. PMID: 27070506
-
Stress-Mediated Enhancement of Ionic Conductivity in Fast-Ion Conductors.ACS Appl Mater Interfaces. 2017 Nov 8;9(44):38773-38783. doi: 10.1021/acsami.7b11687. Epub 2017 Oct 24. ACS Appl Mater Interfaces. 2017. PMID: 29035028
-
Materials with Giant Mechanocaloric Effects: Cooling by Strength.Adv Mater. 2017 Mar;29(11). doi: 10.1002/adma.201603607. Epub 2016 Dec 27. Adv Mater. 2017. PMID: 28026063 Review.
-
Mechanocaloric effects in shape memory alloys.Philos Trans A Math Phys Eng Sci. 2016 Aug 13;374(2074):20150310. doi: 10.1098/rsta.2015.0310. Philos Trans A Math Phys Eng Sci. 2016. PMID: 27402931 Free PMC article. Review.
Cited by
-
Room-temperature mechanocaloric effects in lithium-based superionic materials.Nat Commun. 2018 Aug 20;9(1):3337. doi: 10.1038/s41467-018-05835-9. Nat Commun. 2018. PMID: 30127398 Free PMC article.
-
Colossal barocaloric effects near room temperature in plastic crystals of neopentylglycol.Nat Commun. 2019 Apr 18;10(1):1803. doi: 10.1038/s41467-019-09730-9. Nat Commun. 2019. PMID: 31000715 Free PMC article.
-
Colossal Reversible Barocaloric Effects in a Plastic Crystal Mediated by Lattice Vibrations and Ion Diffusion.Adv Sci (Weinh). 2024 Jul;11(26):e2306488. doi: 10.1002/advs.202306488. Epub 2024 May 5. Adv Sci (Weinh). 2024. PMID: 38704680 Free PMC article.
-
Giant barocaloric effects over a wide temperature range in superionic conductor AgI.Nat Commun. 2017 Nov 29;8(1):1851. doi: 10.1038/s41467-017-01898-2. Nat Commun. 2017. PMID: 29184055 Free PMC article.
-
Colossal barocaloric effects in the complex hydride Li[Formula: see text]B[Formula: see text]H[Formula: see text].Sci Rep. 2021 Jun 7;11(1):11915. doi: 10.1038/s41598-021-91123-4. Sci Rep. 2021. PMID: 34099742 Free PMC article.
References
-
- Goetzler, W., Zogg, R., Young, J. & Johnson, C. Navigant Consulting Inc. (prepared for the U.S. Department of Energy) (Burlington, MA, 2014).
-
- Tušek J, Engelbrecht K, Mañosa Ll, Vives E, Pryds N. Understanding the thermodynamic properties of the elastocaloric effect through experimentation and modelling. Shap. Mem. Superelasticity. 2016;2:317. doi: 10.1007/s40830-016-0094-8. - DOI
-
- Cui J, et al. Demonstration of high efficiency elastocaloric cooling with large ΔT using NiTi wires. Appl. Phys. Lett. 2012;101:073904. doi: 10.1063/1.4746257. - DOI
-
- Waitz T, Tsuchiya K, Antretter T, Fischer FD. MRS Bull. 2009. Phase transformations of nanocrystalline martensitic materials; p. 814.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

