Cis P-tau is induced in clinical and preclinical brain injury and contributes to post-injury sequelae
- PMID: 29042562
- PMCID: PMC5645414
- DOI: 10.1038/s41467-017-01068-4
Cis P-tau is induced in clinical and preclinical brain injury and contributes to post-injury sequelae
Abstract
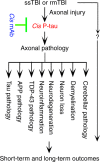
Traumatic brain injury (TBI) is characterized by acute neurological dysfunction and associated with the development of chronic traumatic encephalopathy (CTE) and Alzheimer's disease. We previously showed that cis phosphorylated tau (cis P-tau), but not the trans form, contributes to tau pathology and functional impairment in an animal model of severe TBI. Here we found that in human samples obtained post TBI due to a variety of causes, cis P-tau is induced in cortical axons and cerebrospinal fluid and positively correlates with axonal injury and clinical outcome. Using mouse models of severe or repetitive TBI, we showed that cis P-tau elimination with a specific neutralizing antibody administered immediately or at delayed time points after injury, attenuates the development of neuropathology and brain dysfunction during acute and chronic phases including CTE-like pathology and dysfunction after repetitive TBI. Thus, cis P-tau contributes to short-term and long-term sequelae after TBI, but is effectively neutralized by cis antibody treatment.Induction of the cis form of phosphorylated tau (cis P-tau) has previously been shown to occur in animal models of traumatic brain injury (TBI), and blocking this form of tau using antibody was beneficial in a rodent model of severe TBI. Here the authors show that cis P-tau induction is a feature of several different forms of TBI in humans, and that administration of cis P-tau targeting antibody to rodents reduces or delays pathological features of TBI.
Conflict of interest statement
Dr Lu and Dr Zhou are inventors of Pin1 technology including patents and patent application on tau antibodies, which was licensed by BIDMC to Pinteon Therapeutics. Both Dr Lu and Dr Zhou own equity in, and consult for, Pinteon. Their interests were reviewed and are managed by BIDMC in accordance with its conflict of interest policy. The remaining authors declare no competing financial interests.
Figures

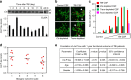
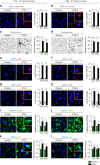
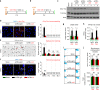


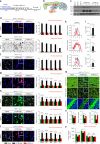

References
-
- Faul, M., Xu, L., Wald, M. M. & Coronado, V. G. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths, 2002–2006. Centers for Disease Control and Prevention [online], http://www.cdc.gov/traumaticbraininjury/tbi_ed.html (2010).
-
- Center for Disease Control Nonfatal traumatic brain injuries from sports and recreation activities --- United States, 2001--2005. MMWR. 2007;56:733–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

