A Novel Technique for Accelerated Culture of Murine Mesenchymal Stem Cells that Allows for Sustained Multipotency
- PMID: 29042571
- PMCID: PMC5645326
- DOI: 10.1038/s41598-017-13477-y
A Novel Technique for Accelerated Culture of Murine Mesenchymal Stem Cells that Allows for Sustained Multipotency
Abstract
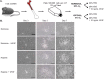
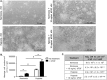
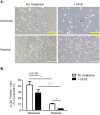
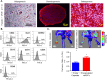
Bone marrow derived mesenchymal stem cells (MSCs) are regularly utilized for translational therapeutic strategies including cell therapy, tissue engineering, and regenerative medicine and are frequently used in preclinical mouse models for both mechanistic studies and screening of new cell based therapies. Current methods to culture murine MSCs (mMSCs) select for rapidly dividing colonies and require long-term expansion. These methods thus require months of culture to generate sufficient cell numbers for feasibility studies in a lab setting and the cell populations often have reduced proliferation and differentiation potential, or have become immortalized cells. Here we describe a simple and reproducible method to generate mMSCs by utilizing hypoxia and basic fibroblast growth factor supplementation. Cells produced using these conditions were generated 2.8 times faster than under traditional methods and the mMSCs showed decreased senescence and maintained their multipotency and differentiation potential until passage 11 and beyond. Our method for mMSC isolation and expansion will significantly improve the utility of this critical cell source in pre-clinical studies for the investigation of MSC mechanisms, therapies, and cell manufacturing strategies.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Phinney DG. Isolation of mesenchymal stem cells from murine bone marrow by immunodepletion. Methods Mol Biol. 2008;449:171–186. - PubMed
-
- Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999;72:570–585. doi: 10.1002/(SICI)1097-4644(19990315)72:4<570::AID-JCB12>3.0.CO;2-W. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

