Immunoproteasome subunit ß5i/LMP7-deficiency in atherosclerosis
- PMID: 29042581
- PMCID: PMC5645401
- DOI: 10.1038/s41598-017-13592-w
Immunoproteasome subunit ß5i/LMP7-deficiency in atherosclerosis
Abstract
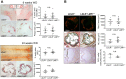
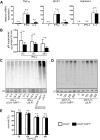
Management of protein homeostasis by the ubiquitin-proteasome system is critical for atherosclerosis development. Recent studies showed controversial results on the role of immunoproteasome (IP) subunit β5i/LMP7 in maintenance of protein homeostasis under cytokine induced oxidative stress. The present study aimed to investigate the effect of β5i/LMP7-deficiency on the initiation and progression of atherosclerosis as a chronic inflammatory, immune cell driven disease. LDLR-/-LMP7-/- and LDLR-/- mice were fed a Western-type diet for either 6 or 24 weeks to induce early and advanced stage atherosclerosis, respectively. Lesion burden was similar between genotypes in both stages. Macrophage content and abundance of polyubiquitin conjugates in aortic root plaques were unaltered by β5i/LMP7-deficiency. In vitro experiments using bone marrow-derived macrophages (BMDM) showed that β5i/LMP7-deficiency did not influence macrophage polarization or accumulation of polyubiquitinated proteins and cell survival upon hydrogen peroxide and interferon-γ treatment. Analyses of proteasome core particle composition by Western blot revealed incorporation of standard proteasome subunits in β5i/LMP7-deficient BMDM and spleen. Chymotrypsin-, trypsin- and caspase-like activities assessed by using short fluorogenic peptides in BMDM whole cell lysates were similar in both genotypes. Taken together, deficiency of IP subunit β5i/LMP7 does not disturb protein homeostasis and does not aggravate atherogenesis in LDLR-/- mice.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Marfella R, et al. Increased activity of the ubiquitin-proteasome system in patients with symptomatic carotid disease is associated with enhanced inflammation and may destabilize the atherosclerotic plaque: effects of rosiglitazone treatment. Journal of the American College of Cardiology. 2006;47:2444–2455. doi: 10.1016/j.jacc.2006.01.073. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

