Abnormal M1/M2 macrophage phenotype profiles in the small airway wall and lumen in smokers and chronic obstructive pulmonary disease (COPD)
- PMID: 29042607
- PMCID: PMC5645352
- DOI: 10.1038/s41598-017-13888-x
Abnormal M1/M2 macrophage phenotype profiles in the small airway wall and lumen in smokers and chronic obstructive pulmonary disease (COPD)
Abstract
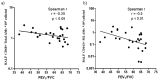
We explore potential dysregulation of macrophage phenotypes in COPD pathogenesis through integrated study of human small airway tissue, bronchoalveolar lavage (BAL) and an experimental murine model of COPD. We evaluated human airway tissue and BAL from healthy controls, normal lung function smokers (NLFS), and COPD subjects. Both small airways and BAL cells were immunohistochemically stained with anti-CD68 for total macrophages and with anti-CD163 for M2, and anti-iNOS for M1 macrophages. Multiplex ELISA measured BAL cytokines. Comparable cigarette smoke-induced experimental COPD mouse model was assessed for relevant mRNA profiles. We found an increase in pro-inflammatory M1s in the small airways of NLFS and COPD compared to controls with a reciprocal decrease in M2 macrophages, which remained unchanged among pathological groups. However, luminal macrophages showed a dominant M2 phenotype in both NLFS and COPD subjects. BAL cytokine skewed towards an M2 profile with increase in CCL22, IL-4, IL-13, and IL-10 in both NLFS and COPDs. The mouse-model of COPD showed similar increase in mRNA for M2 markers. Our finding suggests abnormal macrophage switching in both mucosal and luminal areas of COPD patients, that strongly associated with cytokine balance. There may be potential for beneficial therapeutic cytokine manipulation of macrophage phenotypes in COPD.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


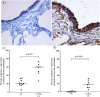
 ) Dual stained CD68 + iNOS + cells, (
) Dual stained CD68 + iNOS + cells, ( ) only CD68 + cells. CD163 staining M2 macrophages (c) NC (d) COPD-CS.
) only CD68 + cells. CD163 staining M2 macrophages (c) NC (d) COPD-CS.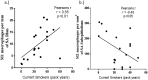






References
-
- Hogg, J. C. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. The Lancet364, 709-721, 10.1016/S0140-6736 (04)16900-6. - PubMed
-
- Eapen, M. S., Myers, S., Walters, E. H. & Sohal, S. S. Airway inflammation in chronic obstructive pulmonary disease (COPD): a true paradox. Expert review of respiratory medicine, 1-13, 10.1080/17476348.2017.1360769 (2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

