HIV-1 viral protein R (Vpr) induces fatty liver in mice via LXRα and PPARα dysregulation: implications for HIV-specific pathogenesis of NAFLD
- PMID: 29042644
- PMCID: PMC5645472
- DOI: 10.1038/s41598-017-13835-w
HIV-1 viral protein R (Vpr) induces fatty liver in mice via LXRα and PPARα dysregulation: implications for HIV-specific pathogenesis of NAFLD
Abstract
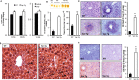
HIV patients develop hepatic steatosis. We investigated hepatic steatosis in transgenic mice expressing the HIV-1 accessory protein Vpr (Vpr-Tg) in liver and adipose tissues, and WT mice infused with synthetic Vpr. Vpr-Tg mice developed increased liver triglyceride content and elevated ALT, bilirubin and alkaline phosphatase due to three hepatic defects: 1.6-fold accelerated de novo lipogenesis (DNL), 45% slower fatty acid ß-oxidation, and 40% decreased VLDL-triglyceride export. Accelerated hepatic DNL was due to coactivation by Vpr of liver X receptor-α (LXRα) with increased expression of its lipogenic targets Srebp1c, Chrebp, Lpk, Dgat, Fasn and Scd1, and intranuclear SREBP1c and ChREBP. Vpr enhanced association of LXRα with Lxrα and Srebp1c promoters, increased LXRE-LXRα binding, and broadly altered hepatic expression of LXRα-regulated lipid metabolic genes. Diminished hepatic fatty acid ß-oxidation was associated with decreased mRNA expression of Pparα and its targets Cpt1, Aox, Lcad, Ehhadh, Hsd10 and Acaa2, and blunted VLDL export with decreased expression of Mttp and its product microsomal triglyceride transfer protein. With our previous findings that Vpr circulates in HIV patients (including those with undetectable plasma HIV-1 RNA), co-regulates the glucocorticoid receptor and PPARγ and transduces hepatocytes, these data indicate a potential role for Vpr in HIV-associated fatty liver disease.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
HIV-1 Vpr induces adipose dysfunction in vivo through reciprocal effects on PPAR/GR co-regulation.Sci Transl Med. 2013 Nov 27;5(213):213ra164. doi: 10.1126/scitranslmed.3007148. Sci Transl Med. 2013. PMID: 24285483 Free PMC article.
-
Methionine restriction prevents the progression of hepatic steatosis in leptin-deficient obese mice.Metabolism. 2013 Nov;62(11):1651-61. doi: 10.1016/j.metabol.2013.06.012. Epub 2013 Aug 5. Metabolism. 2013. PMID: 23928105
-
Histone H3K9 Demethylase JMJD2B Plays a Role in LXRα-Dependent Lipogenesis.Int J Mol Sci. 2020 Nov 5;21(21):8313. doi: 10.3390/ijms21218313. Int J Mol Sci. 2020. PMID: 33167594 Free PMC article.
-
Hepatic lipid accumulation: cause and consequence of dysregulated glucoregulatory hormones.J Endocrinol. 2017 Jul;234(1):R1-R21. doi: 10.1530/JOE-16-0513. Epub 2017 Apr 20. J Endocrinol. 2017. PMID: 28428362 Review.
-
Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease.J Hepatol. 2015 Mar;62(3):720-33. doi: 10.1016/j.jhep.2014.10.039. Epub 2014 Nov 1. J Hepatol. 2015. PMID: 25450203 Review.
Cited by
-
Pathophysiology and Clinical Management of Dyslipidemia in People Living with HIV: Sailing through Rough Seas.Life (Basel). 2024 Mar 28;14(4):449. doi: 10.3390/life14040449. Life (Basel). 2024. PMID: 38672720 Free PMC article. Review.
-
Examining Relationships between Metabolism and Persistent Inflammation in HIV Patients on Antiretroviral Therapy.Mediators Inflamm. 2018 Sep 27;2018:6238978. doi: 10.1155/2018/6238978. eCollection 2018. Mediators Inflamm. 2018. PMID: 30363715 Free PMC article. Review.
-
Performance of an automated deep learning algorithm to identify hepatic steatosis within noncontrast computed tomography scans among people with and without HIV.Pharmacoepidemiol Drug Saf. 2023 Oct;32(10):1121-1130. doi: 10.1002/pds.5648. Epub 2023 Jun 8. Pharmacoepidemiol Drug Saf. 2023. PMID: 37276449 Free PMC article.
-
Adipose Tissue Dysfunction and Energy Balance Paradigms in People Living With HIV.Endocr Rev. 2024 Mar 4;45(2):190-209. doi: 10.1210/endrev/bnad028. Endocr Rev. 2024. PMID: 37556371 Free PMC article. Review.
-
HIV Persistence in Adipose Tissue Reservoirs.Curr HIV/AIDS Rep. 2018 Feb;15(1):60-71. doi: 10.1007/s11904-018-0378-z. Curr HIV/AIDS Rep. 2018. PMID: 29423731 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

