MG-132 reduces virus release in Bovine herpesvirus-1 infection
- PMID: 29042667
- PMCID: PMC5645422
- DOI: 10.1038/s41598-017-13717-1
MG-132 reduces virus release in Bovine herpesvirus-1 infection
Abstract
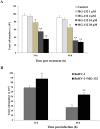
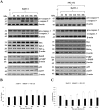
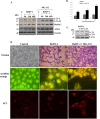
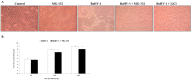
Bovine herpesvirus 1 (BoHV-1) can provoke conjunctivitis, abortions and shipping fever. BoHV-1 infection can also cause immunosuppression and increased susceptibility to secondary bacterial infections, leading to pneumonia and occasionally to death. Herein, we investigated the influence of MG-132, a proteasome inhibitor, on BoHV-1 infection in bovine kidney (MDBK) cells. Infection of MDBK cells with BoHV-1 induces apoptotic cell death that enhances virus release. Whereas, MG-132 inhibited virus-induced apoptosis and stimulated autophagy. Protein expression of viral infected cell protein 0 (bICP0), which is constitutively expressed during infection and is able to stimulate Nuclear factor kappa B (NF-κB), was completely inhibited by MG-132. These results were accompanied by a significant delay in the NF-κB activation. Interestingly, the efficient virus release provoked by BoHV-1-induced apoptosis was significantly reduced by MG-132. Overall, this study suggests that MG-132, through the activation of autophagy, may limit BoHV-1 replication during productive infection, by providing an antiviral defense mechanism.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Combinatorial Effects of the Glucocorticoid Receptor and Krüppel-Like Transcription Factor 15 on Bovine Herpesvirus 1 Transcription and Productive Infection.J Virol. 2017 Oct 13;91(21):e00904-17. doi: 10.1128/JVI.00904-17. Print 2017 Nov 1. J Virol. 2017. PMID: 28794031 Free PMC article.
-
Bovine herpesvirus-1 infection in mouse neuroblastoma (Neuro-2A) cells.Vet Microbiol. 2020 Aug;247:108762. doi: 10.1016/j.vetmic.2020.108762. Epub 2020 Jun 15. Vet Microbiol. 2020. PMID: 32768214
-
Bovine Herpesvirus 1 Counteracts Immune Responses and Immune-Surveillance to Enhance Pathogenesis and Virus Transmission.Front Immunol. 2019 May 7;10:1008. doi: 10.3389/fimmu.2019.01008. eCollection 2019. Front Immunol. 2019. PMID: 31134079 Free PMC article. Review.
-
Progesterone Sporadically Induces Reactivation from Latency in Female Calves but Proficiently Stimulates Bovine Herpesvirus 1 Productive Infection.J Virol. 2022 Mar 9;96(5):e0213021. doi: 10.1128/jvi.02130-21. Epub 2022 Jan 12. J Virol. 2022. PMID: 35019726 Free PMC article.
-
Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis.Vet Res. 2007 Mar-Apr;38(2):181-209. doi: 10.1051/vetres:2006059. Epub 2007 Jan 25. Vet Res. 2007. PMID: 17257569 Review.
Cited by
-
Several Alphaherpesviruses Interact Similarly with the NF-κB Pathway and Suppress NF-κB-Dependent Gene Expression.Microbiol Spectr. 2023 Aug 17;11(4):e0142123. doi: 10.1128/spectrum.01421-23. Epub 2023 Jul 19. Microbiol Spectr. 2023. PMID: 37466427 Free PMC article.
-
Glycyrrhizin alleviates BoAHV-1-induced lung injury in guinea pigs by inhibiting the NF-κB/NLRP3 Signaling pathway and activating the Nrf2/HO-1 Signaling pathway.Vet Res Commun. 2024 Aug;48(4):2499-2511. doi: 10.1007/s11259-024-10436-7. Epub 2024 Jun 12. Vet Res Commun. 2024. PMID: 38865040
-
Bovine Herpesvirus 1 Entry by a Low-pH Endosomal Pathway.J Virol. 2018 Sep 26;92(20):e00839-18. doi: 10.1128/JVI.00839-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30045989 Free PMC article.
-
2,3,7,8-Tetrachlorodibenzo-p-dioxin influences bovine herpesvirus 1 replication through upregulation of SIRT3 and cytoskeletal reorganization.Vet Res Commun. 2017 Dec;41(4):299-306. doi: 10.1007/s11259-017-9701-1. Epub 2017 Oct 28. Vet Res Commun. 2017. PMID: 29081026 Free PMC article.
-
The Involvement of Histone H3 Acetylation in Bovine Herpesvirus 1 Replication in MDBK Cells.Viruses. 2018 Sep 27;10(10):525. doi: 10.3390/v10100525. Viruses. 2018. PMID: 30261679 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

