RNA editing by ADAR1 regulates innate and antiviral immune functions in primary macrophages
- PMID: 29042669
- PMCID: PMC5645456
- DOI: 10.1038/s41598-017-13580-0
RNA editing by ADAR1 regulates innate and antiviral immune functions in primary macrophages
Abstract
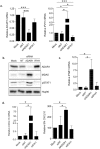
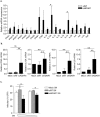
ADAR1-dependent A-to-I editing has recently been recognized as a key process for marking dsRNA as self, therefore, preventing innate immune activation and affecting the development and resolution of immune-mediated diseases and infections. Here, we have determined the role of ADAR1 as a regulator of innate immune activation and modifier of viral susceptibility in primary myeloid and lymphoid cells. We show that ADAR1 knockdown significantly enhanced interferon, cytokine and chemokine production in primary macrophages that function as antiviral paracrine factors, rendering them resistant to HIV-1 infection. ADAR1 knockdown induced deregulation of the RLRs-MAVS signaling pathway, by increasing MDA5, RIG-I, IRF7 and phospho-STAT1 expression, an effect that was partially rescued by pharmacological blockade of the pathway. In summary, our results demonstrate a role of ADAR1 in regulating innate immune function in primary macrophages, suggesting that macrophages may play an essential role in disease associated to ADAR1 dysfunction. We also show that viral inhibition is exclusively dependent on innate immune activation consequence of ADAR1 knockdown, pointing towards ADAR1 as a potential target to boost antiviral immune response.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
ADAR1 affects HCV infection by modulating innate immune response.Antiviral Res. 2018 Aug;156:116-127. doi: 10.1016/j.antiviral.2018.05.012. Epub 2018 Jun 12. Antiviral Res. 2018. PMID: 29906476
-
Isoforms of RNA-Editing Enzyme ADAR1 Independently Control Nucleic Acid Sensor MDA5-Driven Autoimmunity and Multi-organ Development.Immunity. 2015 Nov 17;43(5):933-44. doi: 10.1016/j.immuni.2015.11.001. Immunity. 2015. PMID: 26588779 Free PMC article.
-
ADAR1 interaction with Z-RNA promotes editing of endogenous double-stranded RNA and prevents MDA5-dependent immune activation.Cell Rep. 2021 Aug 10;36(6):109500. doi: 10.1016/j.celrep.2021.109500. Cell Rep. 2021. PMID: 34380029
-
ADAR1, inosine and the immune sensing system: distinguishing self from non-self.Wiley Interdiscip Rev RNA. 2016 Mar-Apr;7(2):157-72. doi: 10.1002/wrna.1322. Epub 2015 Dec 21. Wiley Interdiscip Rev RNA. 2016. PMID: 26692549 Review.
-
The role of RNA editing by ADAR1 in prevention of innate immune sensing of self-RNA.J Mol Med (Berl). 2016 Oct;94(10):1095-1102. doi: 10.1007/s00109-016-1416-1. Epub 2016 Apr 5. J Mol Med (Berl). 2016. PMID: 27044320 Review.
Cited by
-
MicroRNAs are deeply linked to the emergence of the complex octopus brain.Sci Adv. 2022 Nov 25;8(47):eadd9938. doi: 10.1126/sciadv.add9938. Epub 2022 Nov 25. Sci Adv. 2022. PMID: 36427315 Free PMC article.
-
ADAR regulates APOL1 via A-to-I RNA editing by inhibition of MDA5 activation in a paradoxical biological circuit.Proc Natl Acad Sci U S A. 2022 Nov;119(44):e2210150119. doi: 10.1073/pnas.2210150119. Epub 2022 Oct 25. Proc Natl Acad Sci U S A. 2022. PMID: 36282916 Free PMC article.
-
The Emerging Role of RNA Modifications in the Regulation of Antiviral Innate Immunity.Front Microbiol. 2022 Feb 3;13:845625. doi: 10.3389/fmicb.2022.845625. eCollection 2022. Front Microbiol. 2022. PMID: 35185855 Free PMC article. Review.
-
Host-directed editing of the SARS-CoV-2 genome.Biochem Biophys Res Commun. 2021 Jan 29;538:35-39. doi: 10.1016/j.bbrc.2020.10.092. Epub 2020 Nov 5. Biochem Biophys Res Commun. 2021. PMID: 33234239 Free PMC article. Review.
-
Deciphering the multifaceted role of double-stranded RNA sensor protein kinase R: pathophysiological function beyond the antiviral response.RNA Biol. 2025 Dec;22(1):1-14. doi: 10.1080/15476286.2025.2512610. Epub 2025 May 30. RNA Biol. 2025. PMID: 40444707 Free PMC article. Review.
References
-
- George, C. X., John, L. & Samuel, C. E. An RNA editor, adenosine deaminase acting on double-stranded RNA (ADAR1). Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research 34, 437–446, 10.1089/jir.2014.0001 (2014). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

