Decreased frequency of adenoidectomy by a 12-week nasal budesonide treatment
- PMID: 29042788
- PMCID: PMC5633319
- DOI: 10.2147/TCRM.S144659
Decreased frequency of adenoidectomy by a 12-week nasal budesonide treatment
Abstract
Objective: There is little evidence on the role of topical budesonide in reducing the frequency of adenoidectomy, although it was reported that topical budesonide can effectively ameliorate the symptoms of adenoid hypertrophy (AH). This study was aimed to investigate the possibility and safety of alternatives to adenoidectomy with a 12-week treatment with nasal budesonide.
Materials and methods: One hundred patients with AH were randomized to receive either a double-blind budesonide (1 mg once daily) or placebo treatment for 2 weeks by transnasal nebulization. A further 12-week open study, budesonide spray (64 μg per nostril at bedtime) was administered to the treatment group. During the final 12 weeks of follow-up, the frequency of adenotonsillectomy, side effects, the degree of nasal obstruction, nasal discharge, and snoring were assessed.
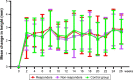
Results: Out of the 100 total enrolled patients, 92 children with AH completed the study. After the 2-week treatment with transnasal budesonide nebulization, the symptoms of AH significantly decreased compared to the control group. Responders (n=26) who had initially improved showed significantly decreased symptoms of AH, and the frequency of adenotonsillectomy during the follow-up (14 and 26 weeks) was compared with that of the control group and non-responders (n=21) who did not respond to the initial 2-week budesonide therapy. The 12-week nasal budesonide treatment did not suppress the growth rate of children's height or cause other side effects.
Conclusion: AH children who had improved after an initial 2-week budesonide therapy can achieve clinical improvements and decreased frequency of adenoidectomy following the therapy with a 12-week treatment with nasal budesonide.
Keywords: adenoid hypertrophy; adenoidectomy; budesonide; frequency; treatment.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
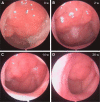
Figures


Similar articles
-
[Efficacy and safety assessment of transnasal nebulisation of budesonide in children with adenoid hypertrophy].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2024 Dec;38(12):1154-1160. doi: 10.13201/j.issn.2096-7993.2024.12.012. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2024. PMID: 39605266 Free PMC article. Clinical Trial. Chinese.
-
[The efficacy and safety of budesonide inhalation suspension via transnasal nebulization compared with oral corticosteroids in chronic rhinosinusitis with nasal polyps].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015 May;29(9):792-6. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015. PMID: 26281053 Clinical Trial. Chinese.
-
Pediatric adenoidal hypertrophy and nasal airway obstruction: reduction with aqueous nasal beclomethasone.Pediatrics. 1995 Mar;95(3):355-64. Pediatrics. 1995. PMID: 7862473 Clinical Trial.
-
Efficacy of nasal corticosteroid in preventing regrowth after adenoidectomy.Auris Nasus Larynx. 2016 Dec;43(6):637-40. doi: 10.1016/j.anl.2016.02.001. Epub 2016 Mar 29. Auris Nasus Larynx. 2016. PMID: 27036360 Clinical Trial.
-
Once-daily budesonide aqueous nasal spray for allergic rhinitis: a review.Clin Ther. 2004 Apr;26(4):473-92. doi: 10.1016/s0149-2918(04)90050-1. Clin Ther. 2004. PMID: 15189745 Review.
Cited by
-
Update of endoscopic classification system of adenoid hypertrophy based on clinical experience on 7621 children.Acta Otorhinolaryngol Ital. 2022 Jun;42(3):257-264. doi: 10.14639/0392-100X-N1832. Epub 2022 Apr 8. Acta Otorhinolaryngol Ital. 2022. PMID: 35396589 Free PMC article.
-
Intranasal Corticosteroids and Oral Montelukast for Paediatric Obstructive Sleep Apnoea: A Systematic Review.Pharmaceutics. 2025 Apr 30;17(5):588. doi: 10.3390/pharmaceutics17050588. Pharmaceutics. 2025. PMID: 40430879 Free PMC article. Review.
-
Inter-society consensus for the use of inhaled corticosteroids in infants, children and adolescents with airway diseases.Ital J Pediatr. 2021 Apr 21;47(1):97. doi: 10.1186/s13052-021-01013-8. Ital J Pediatr. 2021. PMID: 33882987 Free PMC article.
-
[Efficacy and safety assessment of transnasal nebulisation of budesonide in children with adenoid hypertrophy].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2024 Dec;38(12):1154-1160. doi: 10.13201/j.issn.2096-7993.2024.12.012. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2024. PMID: 39605266 Free PMC article. Clinical Trial. Chinese.
-
Numerical modelling of micron particle inhalation in a realistic nasal airway with pediatric adenoid hypertrophy: A virtual comparison between pre- and postoperative models.Front Pediatr. 2023 Feb 23;11:1083699. doi: 10.3389/fped.2023.1083699. eCollection 2023. Front Pediatr. 2023. PMID: 36911037 Free PMC article.
References
-
- Kaditis AG, Alonso AM, Boudewyns A, et al. Obstructive sleep disordered breathing in 2- to 18-year-old children: diagnosis and management. Eur Respir J. 2016;47:69–94. - PubMed
-
- Baugh RF, Archer SM, Mitchell RB, et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144:S1–S30. - PubMed
-
- Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin North Am. 1989;36:1551–1569. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

