Feeding filaggrin: effects of l-histidine supplementation in atopic dermatitis
- PMID: 29042806
- PMCID: PMC5634381
- DOI: 10.2147/CCID.S146760
Feeding filaggrin: effects of l-histidine supplementation in atopic dermatitis
Abstract
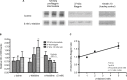
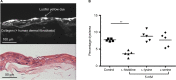
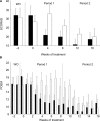
Atopic dermatitis (AD), also known as eczema, is one of the most common chronic skin conditions worldwide, affecting up to 16% of children and 10% of adults. It is incurable and has significant psychosocial and economic impacts on the affected individuals. AD etiology has been linked to deficiencies in the skin barrier protein, filaggrin. In mammalian skin, l-histidine is rapidly incorporated into filaggrin. Subsequent filaggrin proteolysis releases l-histidine as an important natural moisturizing factor (NMF). In vitro studies were conducted to investigate the influence of l-histidine on filaggrin processing and barrier function in human skin-equivalent models. Our further aim was to examine the effects of daily oral l-histidine supplementation on disease severity in adult AD patients. We conducted a randomized, double-blind, placebo-controlled, crossover, nutritional supplementation pilot study to explore the effects of oral l-histidine in adult AD patients (n=24). In vitro studies demonstrated that l-histidine significantly increased both filaggrin formation and skin barrier function (P<0.01, respectively). Data from the clinical study indicated that once daily oral l-histidine significantly reduced (P<0.003) AD disease severity by 34% (physician assessment using the SCORingAD tool) and 39% (patient self-assessment using the Patient Oriented Eczema Measure tool) after 4 weeks of treatment. No improvement was noted with the placebo (P>0.32). The clinical effect of oral l-histidine in AD was similar to that of mid-potency topical corticosteroids and combined with its safety profile suggests that it may be a safe, nonsteroidal approach suitable for long-term use in skin conditions that are associated with filaggrin deficits such as AD.
Keywords: amino acid; atopic dermatitis; eczema; filaggrin; l-histidine; nutritional supplement; skin barrier.
Conflict of interest statement
Disclosure CEMG reports grants from Zymogenetics, Stiefel, and Regeneron, grants and personal fees from Novartis and Pfizer. NKG is a founding director of Curapel, a University of Manchester spin-out company owning patents in this field. The other authors report no conflicts of interest in this work.
Figures

Similar articles
-
l-Histidine Supplementation in Adults and Young Children with Atopic Dermatitis (Eczema).J Nutr. 2020 Oct 1;150(Suppl 1):2576S-2579S. doi: 10.1093/jn/nxaa200. J Nutr. 2020. PMID: 33000160 Review.
-
Concentration of filaggrin monomers, its metabolites and corneocyte surface texture in individuals with a history of atopic dermatitis and controls.J Eur Acad Dermatol Venereol. 2018 May;32(5):796-804. doi: 10.1111/jdv.14801. Epub 2018 Feb 12. J Eur Acad Dermatol Venereol. 2018. PMID: 29360238
-
Barrier repair therapy in atopic dermatitis: an overview.Am J Clin Dermatol. 2013 Oct;14(5):389-99. doi: 10.1007/s40257-013-0033-9. Am J Clin Dermatol. 2013. PMID: 23757122 Review.
-
Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis.J Eur Acad Dermatol Venereol. 2015 Jun;29(6):1091-5. doi: 10.1111/jdv.12753. Epub 2014 Oct 1. J Eur Acad Dermatol Venereol. 2015. PMID: 25271795
-
Changes in nano-mechanical properties of human epidermal cornified cells in children with atopic dermatitis.Wellcome Open Res. 2020 Jul 17;5:97. doi: 10.12688/wellcomeopenres.15729.2. eCollection 2020. Wellcome Open Res. 2020. PMID: 32954014 Free PMC article.
Cited by
-
Influence of Histidine Administration on Ammonia and Amino Acid Metabolism: A Review.Physiol Res. 2020 Aug 31;69(4):555-564. doi: 10.33549/physiolres.934449. Epub 2020 Jun 25. Physiol Res. 2020. PMID: 32584129 Free PMC article. Review.
-
Effect of Topical Microencapsulated Benzoyl Peroxide on the Skin Microbiome in Rosacea: A Randomized, Double-Blind, Crossover, Vehicle-Controlled Clinical Trial.J Clin Aesthet Dermatol. 2024 Aug;17(8):19-26. J Clin Aesthet Dermatol. 2024. PMID: 39148964 Free PMC article.
-
Enantioselective Discrimination of Histidine by Means of an Achiral Cubane-Bridged Bis-Porphyrin.Langmuir. 2021 Nov 30;37(47):13882-13889. doi: 10.1021/acs.langmuir.1c02377. Epub 2021 Nov 16. Langmuir. 2021. PMID: 34784714 Free PMC article.
-
Diet and Skin Barrier: The Role of Dietary Interventions on Skin Barrier Function.Dermatol Pract Concept. 2021 Jan 29;11(1):e2021132. doi: 10.5826/dpc.1101a132. eCollection 2021 Jan. Dermatol Pract Concept. 2021. PMID: 33614213 Free PMC article. Review.
-
Impact of Ergothioneine, Hercynine, and Histidine on Oxidative Degradation of Hyaluronan and Wound Healing.Polymers (Basel). 2020 Dec 29;13(1):95. doi: 10.3390/polym13010095. Polymers (Basel). 2020. PMID: 33383628 Free PMC article.
References
-
- Williams H, Robertson C, Stewart A, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999;103:125–138. - PubMed
-
- Rönmark EP, Ekerljung L, Lötvall J, et al. Eczema among adults: prevalence, risk factors and relation to airway diseases. Results from a large-scale population survey in Sweden. Br J Dermatol. 2012;166:1301–1308. - PubMed
-
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109–1122. - PubMed
-
- Katoh N. Future perspectives in the treatment of atopic dermatitis. J Dermatol. 2009;36:367–376. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

