Changes in pain and concurrent pain medication use following compounded topical analgesic treatment for chronic pain: 3- and 6-month follow-up results from the prospective, observational Optimizing Patient Experience and Response to Topical Analgesics study
- PMID: 29042810
- PMCID: PMC5634368
- DOI: 10.2147/JPR.S143513
Changes in pain and concurrent pain medication use following compounded topical analgesic treatment for chronic pain: 3- and 6-month follow-up results from the prospective, observational Optimizing Patient Experience and Response to Topical Analgesics study
Abstract
Background: Opioids and other controlled substances prescribed for chronic pain are associated with abuse, addiction, and death, prompting national initiatives to identify safe and effective pain management strategies including topical analgesics.
Methods: This prospective, observational study evaluated changes from baseline in overall mean severity and interference scores on the Brief Pain Inventory scale and the use of concurrent pain medications at 3- and 6-month follow-up assessments in chronic pain patients treated with topical analgesics. Changes in pain severity and interference and medication usage were compared between treated patients and unmatched and matched controls.
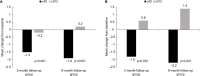
Results: The unmatched intervention group (unmatched-IG) included 631 patients who completed baseline and 3-month follow-up surveys (3-month unmatched-IG) and 158 who completed baseline and 6-month follow-up assessments (6-month unmatched-IG). Baseline and 3-month follow-up data were provided by 76 unmatched controls and 76 matched controls (3-month unmatched-CG and matched-CG), and 51 unmatched and 36 matched patients completed baseline and 6-month follow-up surveys (6-month unmatched-CG and matched-CG). Baseline demographic characteristics and mean pain severity and interference scores were similar between groups. There were statistically significant decreases from baseline in mean pain severity and interference scores within the 3- and 6-month unmatched-IG (all P<0.001). Significantly greater decreases in the mean change from baseline in pain severity and interference scores were evident for the 3- and 6-month unmatched-IG versus unmatched-CG (all P<0.001), with similar results when the 3- and 6-month matched-IG and matched-CG were compared. A higher percentage of the 3- and 6-month unmatched-IG and matched-IG de-escalated use of concurrent pain medications (all P<0.001), while significantly higher percentages of the unmatched-CG and matched-CG escalated medication use. Side effects were reported by <1% of the unmatched-IG.
Conclusion: Topical analgesics appear to be effective and safe for the treatment of chronic pain, with randomized controlled trials needed to confirm these findings.
Keywords: OPERA; chronic pain; opioids; pain interference; pain severity; topical analgesics.
Conflict of interest statement
Disclosure Jeffrey A Gudin and Michael J Brennan have received compensation from Clarity Science for their roles as principal investigators and for providing protocol-required services for the study. E Dennis Harris and Peter L Hurwitz are employees of Clarity Science. James D Strader is the CEO of Safe Harbor Compliance and Clinical Services. Derek T Dietze received compensation for study statistical analyses. The authors report no other conflicts of interest in this work.
Figures




Similar articles
-
Improvement of Pain and Function After Use of a Topical Pain Relieving Patch: Results of the RELIEF Study.J Pain Res. 2020 Jun 26;13:1557-1568. doi: 10.2147/JPR.S258883. eCollection 2020. J Pain Res. 2020. PMID: 32617016 Free PMC article.
-
Reduction of opioid use and improvement in chronic pain in opioid-experienced patients after topical analgesic treatment: an exploratory analysis.Postgrad Med. 2018 Jan;130(1):42-51. doi: 10.1080/00325481.2018.1414551. Postgrad Med. 2018. PMID: 29224410
-
Six-month follow-up of a brief intervention on self-reported safety belt use among emergency department patients.Acad Emerg Med. 2009 Nov;16(11):1221-4. doi: 10.1111/j.1553-2712.2009.00491.x. Epub 2009 Oct 8. Acad Emerg Med. 2009. PMID: 19814758 Clinical Trial.
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
-
Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone).Pain Pract. 2008 Jul-Aug;8(4):287-313. doi: 10.1111/j.1533-2500.2008.00204.x. Epub 2008 May 23. Pain Pract. 2008. PMID: 18503626
Cited by
-
Improvement of Pain and Function After Use of a Topical Pain Relieving Patch: Results of the RELIEF Study.J Pain Res. 2020 Jun 26;13:1557-1568. doi: 10.2147/JPR.S258883. eCollection 2020. J Pain Res. 2020. PMID: 32617016 Free PMC article.
-
Preliminary evidence of safety and effectiveness of Loxoprofen Sodium Cataplasm combined with physiotherapy for myofascial pain syndrome treatment: A randomized controlled pilot clinical trial.Front Neurol. 2022 Nov 22;13:998327. doi: 10.3389/fneur.2022.998327. eCollection 2022. Front Neurol. 2022. PMID: 36484021 Free PMC article.
-
Reducing Opioid Prescriptions by Identifying Responders on Topical Analgesic Treatment Using an Individualized Medicine and Predictive Analytics Approach.J Pain Res. 2020 May 28;13:1255-1266. doi: 10.2147/JPR.S246503. eCollection 2020. J Pain Res. 2020. PMID: 32547186 Free PMC article.
-
MEvA-X: a hybrid multiobjective evolutionary tool using an XGBoost classifier for biomarkers discovery on biomedical datasets.Bioinformatics. 2023 Jul 1;39(7):btad384. doi: 10.1093/bioinformatics/btad384. Bioinformatics. 2023. PMID: 37326976 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

