Cycle control with an extended-regimen oral contraceptive combining levonorgestrel and ethinyl estradiol that includes continuous low-dose ethinyl estradiol instead of the traditional hormone-free interval
- PMID: 29042818
- PMCID: PMC5633331
- DOI: 10.2147/IJWH.S142078
Cycle control with an extended-regimen oral contraceptive combining levonorgestrel and ethinyl estradiol that includes continuous low-dose ethinyl estradiol instead of the traditional hormone-free interval
Abstract
Purpose: To evaluate scheduled and unscheduled bleeding and spotting over 1 year of treatment with 91-day extended-regimen combined oral contraception (COC) providing continuous low-dose ethinyl estradiol (EE) in place of the traditional 7-day hormone-free interval (HFI).
Patients and methods: This post hoc analysis of a multicenter, open-label, 1-year, Phase 3 study of extended-regimen COC with 30 µg EE/150 µg levonorgestrel (LNG) for 84 days and EE 10 µg for 7 days included 799 sexually active, adult women who completed at least one 91-day cycle of therapy. Subjects recorded bleeding and spotting episodes daily using electronic diaries. Logistic regression analyses are reported as ORs with 95% CIs.
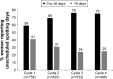
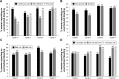
Results: There was a 10% increase (OR =1.102; 95% CI: 1.006-1.206) in the likelihood of reporting no scheduled bleeding for each additional 91-day cycle completed. From the third 91-day cycle, more than one fifth of women reported no scheduled bleeding (third cycle =23% [121/533]; fourth cycle =22% [97/446]). Among women who reported no scheduled bleeding at Cycle 1 (136/758 [18%]), ≥45% showed sustained lack of scheduled bleeding in later cycles. There were increases of 53% (OR =1.531; 95% CI: 1.393-1.683) and 31% (OR =1.307; 95% CI: 1.205-1.418) in the likelihood of reporting 0 to ≤6 days vs >6 days of unscheduled bleeding and spotting, respectively, for each additional 91-day cycle. By Cycle 2, more than 80% of women reported no unscheduled bleeding or ≤6 days of unscheduled bleeding during each 91-day cycle.
Conclusion: Improved cycle control with decreased bleeding over time was shown during extended-regimen COC with 30 µg EE/150 µg LNG for 84 days and continuous low-dose EE instead of the traditional 7-day HFI. Women considering this regimen should be informed that those who complete at least one 91-day COC cycle will likely experience less bleeding/spotting in future cycles.
Keywords: 91-day cycle; combined oral contraception; extended-regimen COC; menstrual cycle.
Conflict of interest statement
Disclosure REN had a financial relationship (lecturer, member of advisory boards, and/or consultant) with Bayer-Schering Pharma, Endoceutics, Gedeon Richter, HRA Pharma, Merck Sharpe & Dohme (MSD), Novo Nordisk, Pfizer Inc, Shionogi Limited, Teva/Theramex, and Zambon SpA. PLA had financial relationship (member of advisory boards, lecturer, and/or consultant) with Bayer, Effik, MSD, Smith & Nephew, and Teva. JH is an employee of Teva Branded Pharmaceutical Products R&D, Inc. MCM is a consultant for Teva Europe Women’s Health Medical Affairs. The authors report no other conflicts of interest in this work.
Figures


Similar articles
-
Efficacy and safety of an ascending-dose, extended-regimen levonorgestrel/ethinyl estradiol combined oral contraceptive.Contraception. 2014 Apr;89(4):299-306. doi: 10.1016/j.contraception.2014.01.013. Epub 2014 Jan 29. Contraception. 2014. PMID: 24576794 Clinical Trial.
-
Oestrogen treatment for increased bleeding in Norplant users: preliminary results.Hum Reprod. 1996 Oct;11 Suppl 2:109-14. doi: 10.1093/humrep/11.suppl_2.109. Hum Reprod. 1996. PMID: 8982752 Clinical Trial.
-
A multicenter, randomized study of an extended cycle oral contraceptive.Contraception. 2003 Aug;68(2):89-96. doi: 10.1016/s0010-7824(03)00141-0. Contraception. 2003. PMID: 12954519 Clinical Trial.
-
Extended-Cycle Levonorgestrel/Ethinylestradiol and Low-Dose Ethinylestradiol (Seasonique(®)): A Review of Its Use as an Oral Contraceptive.Drugs. 2015 Jun;75(9):1019-26. doi: 10.1007/s40265-015-0407-9. Drugs. 2015. PMID: 26017303 Review.
-
Lower hormone dosage with improved cycle control.Eur J Contracept Reprod Health Care. 2002 Dec;7 Suppl 2:25-30; discussion 37-9. Eur J Contracept Reprod Health Care. 2002. PMID: 12659399 Review.
References
-
- Hooper DJ. Attitudes, awareness, compliance and preferences among hormonal contraception users: a global, cross-sectional, self-administered, online survey. Clin Drug Invest. 2010;30(11):749–763. - PubMed
-
- Seval DL, Buckley T, Kuehl TJ, Sulak PJ. Attitudes and prescribing patterns of extended-cycle oral contraceptives. Contraception. 2011;84(1):71–75. - PubMed
-
- Panicker S, Mann S, Shawe J, Stephenson J. Evolution of extended use of the combined oral contraceptive pill. J Fam Plann Reprod Health Care. 2014;40(2):133–141. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

