Inhibition of periderm removal in all-trans retinoic acid-induced cleft palate in mice
- PMID: 29042924
- PMCID: PMC5639390
- DOI: 10.3892/etm.2017.4938
Inhibition of periderm removal in all-trans retinoic acid-induced cleft palate in mice
Abstract
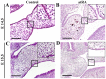
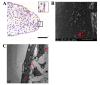
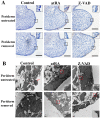
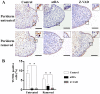
Cleft palate is a common craniofacial birth defect. The aim of the present study was to investigate the effect of excess all-trans retinoic acid (atRA) on periderm removal and the disappearance of basal medial edge epithelial (MEE) cells during palatogenesis, particularly during the stage prior to contact. atRA (200 mg/kg) was administered to C57BL/6N mice at embryonic day (E) 12.0 by gavage. Fetal palates were processed and analyzed by histology and electron microscopy. Single palate shelf peridermal cells were removed and cultured in the presence of atRA (3 µM) only or in the presence of or the caspase inhibitor, Z-VAD (100 µM) only, for 48 h. Once cultured, morphological changes were analyzed by histological staining and electron microscopy. A TUNEL assay was used to detect apoptotic neurons. Paired palatal shelves with periderm removal were cultured in the presence of atRA (3 µM) only or in the presence of Z-VAD (100 µM) only for 48 h and analyzed by hematoxylin and eosin staining. At E14.5, medial edge epithelium periderm was retained in the atRA-treated palates but had been shed prior to contact in the control groups. In addition, atRA was revealed to disrupt the cell cycle in the periderm by downregulating p21. Furthermore, atRA inhibited apoptosis in the periderm and basal MEE cells; however, atRA exhibited no effect on basement membrane degradation in single palatal organ culture. Additionally, once paired palates were cultured for 48 h, all of the groups in which the periderm had been removed exhibited confluence of the embryonic palatal mesenchyme. The present results suggest that periderm removal is inhibited in atRA-induced cleft palate in mice and that removal of the periderm contributes to EPM confluence in vitro.
Keywords: all-trans retinoic acid; cleft palate; p21; periderm.
Figures


Similar articles
-
Activation of Notch1 inhibits medial edge epithelium apoptosis in all-trans retinoic acid-induced cleft palate in mice.Biochem Biophys Res Commun. 2016 Aug 26;477(3):322-8. doi: 10.1016/j.bbrc.2016.06.107. Epub 2016 Jun 23. Biochem Biophys Res Commun. 2016. PMID: 27343556
-
TGFβ3 regulates periderm removal through ΔNp63 in the developing palate.J Cell Physiol. 2015 Jun;230(6):1212-25. doi: 10.1002/jcp.24856. J Cell Physiol. 2015. PMID: 25358290
-
Inhibition of Smad signaling is implicated in cleft palate induced by all-trans retinoic acid.Acta Biol Hung. 2011 Jun;62(2):142-50. doi: 10.1556/ABiol.62.2011.2.4. Acta Biol Hung. 2011. PMID: 21555266
-
Periderm: Life-cycle and function during orofacial and epidermal development.Semin Cell Dev Biol. 2019 Jul;91:75-83. doi: 10.1016/j.semcdb.2017.08.021. Epub 2017 Aug 10. Semin Cell Dev Biol. 2019. PMID: 28803895 Review.
-
Transforming growth factor beta (TGFbeta) signalling in palatal growth, apoptosis and epithelial mesenchymal transformation (EMT).Arch Oral Biol. 2004 Sep;49(9):675-89. doi: 10.1016/j.archoralbio.2004.05.007. Arch Oral Biol. 2004. PMID: 15275855 Review.
Cited by
-
Excess vitamin a might contribute to submucous clefting by inhibiting WNT-mediated bone formation.Orthod Craniofac Res. 2023 Feb;26(1):132-139. doi: 10.1111/ocr.12594. Epub 2022 Jun 28. Orthod Craniofac Res. 2023. PMID: 35716278 Free PMC article.
-
Targeted sequencing reveals KLF4 gene associated with NSCL/P in Western Han Chinese.Int J Clin Exp Pathol. 2019 Oct 1;12(10):3894-3900. eCollection 2019. Int J Clin Exp Pathol. 2019. PMID: 31933779 Free PMC article.
-
Analysis of zebrafish periderm enhancers facilitates identification of a regulatory variant near human KRT8/18.Elife. 2020 Feb 7;9:e51325. doi: 10.7554/eLife.51325. Elife. 2020. PMID: 32031521 Free PMC article.
-
Retinoic acid effects on in vitro palatal fusion and WNT signaling.Eur J Oral Sci. 2022 Dec;130(6):e12899. doi: 10.1111/eos.12899. Epub 2022 Oct 27. Eur J Oral Sci. 2022. PMID: 36303276 Free PMC article.
-
An improved multicellular human organoid model for the study of chemical effects on palatal fusion.Birth Defects Res. 2023 Oct 1;115(16):1513-1533. doi: 10.1002/bdr2.2229. Epub 2023 Aug 2. Birth Defects Res. 2023. PMID: 37530699 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
