Calreticulin is an effective immunologic adjuvant to tumor-associated antigens
- PMID: 29042925
- PMCID: PMC5639401
- DOI: 10.3892/etm.2017.4989
Calreticulin is an effective immunologic adjuvant to tumor-associated antigens
Abstract
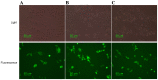
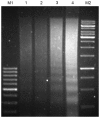
As a key molecule involved in cell recognition, calreticulin (CRT) may be expressed on the surface of (pre-) apoptotic cells and provide the signal that is recognized by dendritic cells (DCs) or other antigen presenting cells (APCs), which results in phagocytosis. Within the APCs, tumor-associated antigens (TAAs) may be subsequently presented to T lymphocytes, which triggers a specific antitumor immune response. It has been hypothesized that CRT is able to act as the immunologic adjuvant and translocate itself and TAAs to the cell surface and induce a specific antitumor immune response. In the present study, CRT was demonstrated to translocate itself and mucin 1 (MUC1), a breast cancer antigen, to the surface of 4T1 cells and the MUC1-CRT-coated cells were able to induce apoptosis in a time-dependent manner. When DCs were infected with adenovirus containing MUC1-CRT, an increase in T cell proliferation and cytokine production was exhibited. These results suggest that CRT may act as an immunologic adjuvant with MUC1 and induce a strong immune response.
Keywords: breast cancer; calreticulin; dendritic cells; mucin 1; tumor-immunity.
Figures



Similar articles
-
Silencing of suppressor of cytokine signaling 1 enhances the immunological effect of mucin 1-calreticulin-primed 4T1 cell-treated dendritic cells in breast cancer treatment.Oncol Lett. 2018 Feb;15(2):1630-1638. doi: 10.3892/ol.2017.7477. Epub 2017 Nov 23. Oncol Lett. 2018. PMID: 29434859 Free PMC article.
-
Breast carcinoma cell lysate-pulsed dendritic cells cross-prime MUC1-specific CD8+ T cells identified by peptide-MHC-class-I tetramers.Cell Immunol. 2004 Sep-Oct;231(1-2):112-25. doi: 10.1016/j.cellimm.2004.12.007. Epub 2005 Feb 8. Cell Immunol. 2004. PMID: 15919376
-
Phenotypic profile of dendritic and T cells in the lymph node of Balb/C mice with breast cancer submitted to dendritic cells immunotherapy.Immunol Lett. 2016 Sep;177:25-37. doi: 10.1016/j.imlet.2016.07.009. Epub 2016 Jul 14. Immunol Lett. 2016. PMID: 27423825
-
Tumor cell lysates as immunogenic sources for cancer vaccine design.Hum Vaccin Immunother. 2014;10(11):3261-9. doi: 10.4161/21645515.2014.982996. Hum Vaccin Immunother. 2014. PMID: 25625929 Free PMC article. Review.
-
Surface-exposed calreticulin in the interaction between dying cells and phagocytes.Ann N Y Acad Sci. 2010 Oct;1209:77-82. doi: 10.1111/j.1749-6632.2010.05740.x. Ann N Y Acad Sci. 2010. PMID: 20958319 Review.
Cited by
-
Novel calreticulin-nanoparticle in combination with focused ultrasound induces immunogenic cell death in melanoma to enhance antitumor immunity.Theranostics. 2020 Feb 10;10(8):3397-3412. doi: 10.7150/thno.42243. eCollection 2020. Theranostics. 2020. PMID: 32206098 Free PMC article.
-
Overcoming Resistance to Immune Checkpoint Inhibitor Therapy Using Calreticulin-Inducing Nanoparticle.Pharmaceutics. 2023 Jun 9;15(6):1693. doi: 10.3390/pharmaceutics15061693. Pharmaceutics. 2023. PMID: 37376141 Free PMC article.
-
Nanomaterials-Based Photodynamic Therapy with Combined Treatment Improves Antitumor Efficacy Through Boosting Immunogenic Cell Death.Int J Nanomedicine. 2021 Jul 8;16:4693-4712. doi: 10.2147/IJN.S314506. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34267518 Free PMC article. Review.
-
Study on the collaborative protective mechanism of Scutellariae Radix and Paeoniae Radix Alba against diabetic cardiomyopathy through the gut-heart axis.Front Microbiol. 2025 Apr 8;16:1500935. doi: 10.3389/fmicb.2025.1500935. eCollection 2025. Front Microbiol. 2025. PMID: 40264972 Free PMC article.
-
Vitamin E succinate exerts anti-tumour effects on human cervical cancer cells via the CD47-SIRPɑ pathway both in vivo and in vitro.J Cancer. 2021 May 5;12(13):3877-3886. doi: 10.7150/jca.52315. eCollection 2021. J Cancer. 2021. PMID: 34093795 Free PMC article.
References
-
- Ahn YH, Hong SO, Kim JH, Noh KH, Song KH, Lee YH, Jeon JH, Kim DW, Seo JH, Kim TW. The siRNA cocktail targeting interleukin 10 receptor and transforming growth factor-β-receptor on dendritic cells potentiates tumor antigen-specific CD8(+) T cell immunity. Clin Exp Immunol. 2015;181:164–178. doi: 10.1111/cei.12620. - DOI - PMC - PubMed
-
- Karbach J, Neumann A, Brand K, Wahle C, Siegel E, Maeurer M, Ritter E, Tsuji T, Gnjatic S, Old LJ, et al. Phase I clinical trial of mixed bacterial vaccine (Coley's toxins) in patients with NY-ESO-1 expressing cancers: Immunological effects and clinical activity. Clin Cancer Res. 2012;18:5449–5459. doi: 10.1158/1078-0432.CCR-12-1116. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
