Expression of LOX-1 in human mesangial cells is increased by Ox-LDL and IL-1β treatment
- PMID: 29042958
- PMCID: PMC5639305
- DOI: 10.3892/etm.2017.4950
Expression of LOX-1 in human mesangial cells is increased by Ox-LDL and IL-1β treatment
Abstract
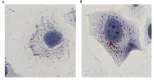
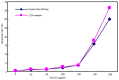
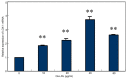
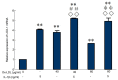
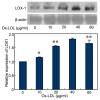
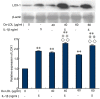
The present study aimed to determine whether the expression of lectin-like oxidized LDL receptor 1 (LOX-1) could be induced by oxidized low-density lipoprotein (Ox-LDL) and interleukin 1β (IL-1β) in human mesangial cells (HMCs). Oil Red O staining was used to observe the uptake of Ox-LDL by HMCs stimulated with IL-1β, and reverse transcription-quantitative polymerase chain reaction analysis and western blotting were used to examine the expression of LOX-1 in HMC following Ox-LDL and IL-1β treatment. Uptake of Ox-LDL by HMCs was upregulated upon stimulation with IL-1β. Furthermore, Ox-LDL (10-40 µg/ml) treatment induced LOX-1 mRNA and protein expression in a dose-dependent manner. In addition, when HMCs were treated with IL-1β and Ox-LDL, the expression of LOX-1 was enhanced further. These results indicated that inhibiting LOX-1 expression or inhibiting the Ox-LDL/LOX-1 signaling axis may be a potential novel method for treating renal disease.
Keywords: human mesangial cell; interleukin 1β; lectin-like oxidized LDL receptor 1; oxidized low-density lipoprotein.
Figures
Similar articles
-
Interleukin-1β Promotes Ox-LDL Uptake by Human Glomerular Mesangial Cells via LOX-1.Int J Med Sci. 2020 Apr 27;17(8):1056-1061. doi: 10.7150/ijms.43981. eCollection 2020. Int J Med Sci. 2020. PMID: 32410835 Free PMC article.
-
Interleukin-1β Regulates Lipid Homeostasis in Human Glomerular Mesangial Cells.J Nutr Health Aging. 2020;24(3):246-250. doi: 10.1007/s12603-019-1302-y. J Nutr Health Aging. 2020. PMID: 32115603
-
[Effects of lecithin-like oxidized low-density lipoprotein receptor-1 on p38 mitogen-activated protein kinase induced by oxidized low-density lipoprotein in endothelial cells].Zhonghua Yi Xue Za Zhi. 2006 Jun 27;86(24):1697-700. Zhonghua Yi Xue Za Zhi. 2006. PMID: 16854325 Chinese.
-
Involvement of LDL and ox-LDL in Cancer Development and Its Therapeutical Potential.Front Oncol. 2022 Feb 16;12:803473. doi: 10.3389/fonc.2022.803473. eCollection 2022. Front Oncol. 2022. PMID: 35251975 Free PMC article. Review.
-
Autoimmune Rheumatic Diseases: An Update on the Role of Atherogenic Electronegative LDL and Potential Therapeutic Strategies.J Clin Med. 2021 May 6;10(9):1992. doi: 10.3390/jcm10091992. J Clin Med. 2021. PMID: 34066436 Free PMC article. Review.
Cited by
-
Association of circulating soluble lectin-like oxidized low-density lipoprotein receptor-1 with inflammatory markers and urinary albumin excretion in patients with type 2 diabetes.SAGE Open Med. 2021 Dec 17;9:20503121211064468. doi: 10.1177/20503121211064468. eCollection 2021. SAGE Open Med. 2021. PMID: 34992779 Free PMC article.
-
[Study on visfatin-induced inflammation and necroptosis via LOX-1 in human umbilical vein endothelial cells].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2020 Oct 25;37(5):834-841. doi: 10.7507/1001-5515.202003067. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2020. PMID: 33140607 Free PMC article. Chinese.
-
Interleukin-1β Promotes Ox-LDL Uptake by Human Glomerular Mesangial Cells via LOX-1.Int J Med Sci. 2020 Apr 27;17(8):1056-1061. doi: 10.7150/ijms.43981. eCollection 2020. Int J Med Sci. 2020. PMID: 32410835 Free PMC article.
References
-
- Li D, Mehta JL. Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implications in apoptosis of human coronary artery endothelial cells: Evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arterioscler Thromb Vasc Biol. 2000;20:1116–1122. doi: 10.1161/01.ATV.20.4.1116. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
