Effect of entecavir in the treatment of patients with hepatitis B virus-related compensated and decompensated cirrhosis
- PMID: 29043000
- PMCID: PMC5639355
- DOI: 10.3892/etm.2017.4963
Effect of entecavir in the treatment of patients with hepatitis B virus-related compensated and decompensated cirrhosis
Abstract
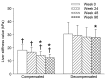
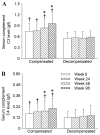
Chronic hepatitis B virus (CHB) infection is a burden on global healthcare and is associated with a higher risk of serious sequelae, including cirrhosis and hepatocellular carcinoma. The clinical application of entecavir as a treatment for CHB has produced positive outcomes, and so is an attractive form of pharmacological therapy. However, little data exists comparing the safety and efficacy of entecavir for the treatment of hepatitis B virus (HBV)-related compensated, and decompensated cirrhosis, respectively. The aim of the present study was to evaluate entecavir therapy as a treatment for patients with HBV-related compensated and decompensated cirrhosis. A retrospective analysis of 46 compensated patients (compensated group) and 51 decompensated cirrhotic patients (decompensated group) treated with entecavir was conducted. Baseline demographics, clinical outcomes, and adverse events during the treatment were compared. Treatment with entecavir for 96 weeks resulted in significant improvements in serum levels of HBV DNA (P=0.002), albumin (P=0.014), cholinesterase (CHE; P=0.001), HBV DNA negativity rate (P=0.004), Child-Turcotte-Pugh score (P=0.030), alanine aminotransferase normalized rate (P=0.039), and the degree of esophageal varices liver stiffness (P=0.002) in the two groups. However, statistical analysis revealed that the improvements were significantly higher in the compensated group compared with the decompensated group (P<0.05). The complement component (C)3 and C4 levels were also significantly increased in the compensated group compared with the decompensated group at weeks 24, 48 and 96 (P<0.05). In addition, the incidences of hepatocellular carcinoma, upper digestive tract hemorrhage and ascites were significantly higher in the decompensated group compared with the compensated group (P<0.05). In conclusion, treatment with 96-week entecavir therapy produced similar clinical outcomes in compensated and decompensated cirrhotic patients via inhibiting HBV-DNA viral load and recovering complement C3 and C4; however, entecavir exerts a better effect on patients with compensated cirrhosis, and so this therapy may improve the prognosis of such patients.
Keywords: cirrhosis; complement; efficacy; entecavir; hepatitis B virus.
Figures
Similar articles
-
Comparison of telbivudine efficacy in treatment-naive patients with hepatitis B virus-related compensated and decompensated cirrhosis in 96 weeks.Eur J Gastroenterol Hepatol. 2014 Apr;26(4):396-403. doi: 10.1097/MEG.0000000000000062. Eur J Gastroenterol Hepatol. 2014. PMID: 24569820
-
Long-term efficacy and safety of lamivudine, entecavir, and tenofovir for treatment of hepatitis B virus-related cirrhosis.Clin Gastroenterol Hepatol. 2013 Jan;11(1):88-94. doi: 10.1016/j.cgh.2012.10.003. Epub 2012 Oct 9. Clin Gastroenterol Hepatol. 2013. PMID: 23063679
-
Prolonged use of tenofovir and entecavir in hepatitis B virus-related cirrhosis.Indian J Gastroenterol. 2015 Jul;34(4):286-91. doi: 10.1007/s12664-015-0576-1. Epub 2015 Aug 6. Indian J Gastroenterol. 2015. PMID: 26243587
-
Treatment of chronic hepatitis B: case selection and duration of therapy.J Gastroenterol Hepatol. 2002 Apr;17(4):409-14. doi: 10.1046/j.1440-1746.2002.02767.x. J Gastroenterol Hepatol. 2002. PMID: 11982721 Review.
-
Lamivudine treatment in patients with chronic hepatitis B and cirrhosis.Expert Opin Pharmacother. 2006 Sep;7(13):1835-43. doi: 10.1517/14656566.7.13.1835. Expert Opin Pharmacother. 2006. PMID: 16925509 Review.
Cited by
-
Comparison of clinical effect of octreotide and pituitrin in treatment of upper gastrointestinal hemorrhage in cirrhosis.Indian J Pharmacol. 2023 Jan-Feb;55(1):21-26. doi: 10.4103/ijp.ijp_653_21. Indian J Pharmacol. 2023. PMID: 36960517 Free PMC article. Clinical Trial.
-
The clinical efficacy and adverse effects of Entecavir plus Thymosin alpha-1 combination therapy versus Entecavir Monotherapy in HBV-related cirrhosis: a systematic review and meta-analysis.BMC Gastroenterol. 2020 Oct 19;20(1):348. doi: 10.1186/s12876-020-01477-8. BMC Gastroenterol. 2020. PMID: 33076834 Free PMC article.
-
Mortality trends in chronic liver disease and cirrhosis from 1981 to 2015 in Taiwan.Popul Health Metr. 2021 Oct 2;19(1):36. doi: 10.1186/s12963-021-00269-w. Popul Health Metr. 2021. PMID: 34600536 Free PMC article.
-
Nucleos(t)ide analogues and Hepatitis B virus-related hepatocellular carcinoma: A literature review.Antivir Chem Chemother. 2020 Jan-Dec;28:2040206620921331. doi: 10.1177/2040206620921331. Antivir Chem Chemother. 2020. PMID: 32418480 Free PMC article. Review.
-
Consensus Guidelines: Best Practices for Detection, Assessment and Management of Suspected Acute Drug-Induced Liver Injury During Clinical Trials in Adults with Chronic Viral Hepatitis and Adults with Cirrhosis Secondary to Hepatitis B, C and Nonalcoholic Steatohepatitis.Drug Saf. 2021 Feb;44(2):133-165. doi: 10.1007/s40264-020-01014-2. Epub 2020 Nov 3. Drug Saf. 2021. PMID: 33141341 Free PMC article. Review.
References
-
- Li Q, Song J, Huang Y, Li X, Zhuo Q, Li W, Chen C, Lu C, Qi X, Chen L. The Gamma-Glutamyl-Transpeptidase to platelet ratio does not show advantages than APRI and Fib-4 in diagnosing significant fibrosis and cirrhosis in patients with Chronic Hepatitis B: A retrospective cohort study in china. Medicine (Baltimore) 2016;95:e3372. doi: 10.1097/MD.0000000000003372. - DOI - PMC - PubMed
-
- Jia W, Qi X, Ji YY, Xun YH, Wang H, Zhang WH, Yang JH, Wang JY, Zhu HX, Mao RC, Zhang JM. Low serum Hepatitis B surface antigen level predicts compensated cirrhosis caused by chronic Hepatitis B in HBeAg positive patients in east china. Hepat Mon. 2015;15:e29183. doi: 10.5812/hepatmon.29183. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
