CNS cell-type localization and LPS response of TLR signaling pathways
- PMID: 29043065
- PMCID: PMC5621151
- DOI: 10.12688/f1000research.12036.1
CNS cell-type localization and LPS response of TLR signaling pathways
Abstract
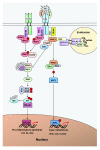
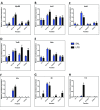
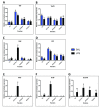
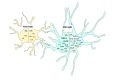
Background: Innate immune signaling in the brain has emerged as a contributor to many central nervous system (CNS) pathologies, including mood disorders, neurodegenerative disorders, neurodevelopmental disorders, and addiction. Toll-like receptors (TLRs), a key component of the innate immune response, are particularly implicated in neuroimmune dysfunction. However, most of our understanding about TLR signaling comes from the peripheral immune response, and it is becoming clear that the CNS immune response is unique. One controversial aspect of neuroimmune signaling is which CNS cell types are involved. While microglia are the CNS cell-type derived from a myeloid lineage, studies suggest that other glial cell types and even neurons express TLRs, although this idea is controversial. Furthermore, recent work suggests a discrepancy between RNA and protein expression within the CNS. Methods: To elucidate the CNS cell-type localization of TLRs and their downstream signaling molecules, we isolated microglia and astrocytes from the brain of adult mice treated with saline or the TLR4 ligand lipopolysaccharide (LPS). Glial mRNA and protein expression was compared to a cellular-admixture to determine cell-type enrichment. Results: Enrichment analysis revealed that most of the TLR pathway genes are localized in microglia and changed in microglia following immune challenge. However, expression of Tlr3 was enriched in astrocytes, where it increased in response to LPS. Furthermore, attempts to determine protein cell-type localization revealed that many antibodies are non-specific and that antibody differences are contributing to conflicting localization results. Conclusions: Together these results highlight the cell types that should be looked at when studying TLR signaling gene expression and suggest that non-antibody approaches need to be used to accurately evaluate protein expression.
Keywords: MyD88; TRIF; Toll-like receptor; astrocyte; lipopolysaccharide; microglia; neuroimmune.
Conflict of interest statement
Competing interests: No competing interests were disclosed.
Figures




Similar articles
-
TLR signaling tailors innate immune responses in human microglia and astrocytes.J Immunol. 2005 Oct 1;175(7):4320-30. doi: 10.4049/jimmunol.175.7.4320. J Immunol. 2005. PMID: 16177072
-
Ethanol, TLR3, and TLR4 Agonists Have Unique Innate Immune Responses in Neuron-Like SH-SY5Y and Microglia-Like BV2.Alcohol Clin Exp Res. 2017 May;41(5):939-954. doi: 10.1111/acer.13368. Epub 2017 Mar 30. Alcohol Clin Exp Res. 2017. PMID: 28273337 Free PMC article.
-
Ligand engagement of Toll-like receptors regulates their expression in cortical microglia and astrocytes.J Neuroinflammation. 2015 Dec 30;12:244. doi: 10.1186/s12974-015-0458-6. J Neuroinflammation. 2015. PMID: 26714634 Free PMC article.
-
Toll-like receptors and inflammation in the CNS.Curr Drug Targets Inflamm Allergy. 2002 Jun;1(2):181-91. doi: 10.2174/1568010023344698. Curr Drug Targets Inflamm Allergy. 2002. PMID: 14561199 Review.
-
Targeting toll-like receptor 4 to modulate neuroinflammation in central nervous system disorders.Expert Opin Ther Targets. 2019 Oct;23(10):865-882. doi: 10.1080/14728222.2019.1676416. Epub 2019 Oct 20. Expert Opin Ther Targets. 2019. PMID: 31580163 Review.
Cited by
-
Neonatal exposure to organophosphorus flame retardant TDCPP elicits neurotoxicity in mouse hippocampus via microglia-mediated inflammation in vivo and in vitro.Arch Toxicol. 2020 Feb;94(2):541-552. doi: 10.1007/s00204-019-02635-y. Epub 2020 Jan 1. Arch Toxicol. 2020. PMID: 31894355
-
Oxytocin, Dopamine, and Opioid Interactions Underlying Pair Bonding: Highlighting a Potential Role for Microglia.Endocrinology. 2021 Feb 1;162(2):bqaa223. doi: 10.1210/endocr/bqaa223. Endocrinology. 2021. PMID: 33367612 Free PMC article. Review.
-
Increased alcohol self-administration following repeated Toll-like receptor 3 agonist treatment in male and female rats.Pharmacol Biochem Behav. 2022 May;216:173379. doi: 10.1016/j.pbb.2022.173379. Epub 2022 Apr 5. Pharmacol Biochem Behav. 2022. PMID: 35395252 Free PMC article.
-
The Toll Pathway in the Central Nervous System of Flies and Mammals.Neuromolecular Med. 2018 Dec;20(4):419-436. doi: 10.1007/s12017-018-8515-9. Epub 2018 Oct 1. Neuromolecular Med. 2018. PMID: 30276585 Review.
-
Study on the Influence and Mechanism of Resveratrol on Cognitive Impairment in Chronic Kidney Disease Rats Through Regulating Gut Microbiota and the TLR4/NFκB Pathway.J Inflamm Res. 2025 May 8;18:6049-6060. doi: 10.2147/JIR.S510867. eCollection 2025. J Inflamm Res. 2025. PMID: 40357381 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

