Randomized study of etirinotecan pegol versus irinotecan as second-line treatment for metastatic colorectal cancer
- PMID: 29043412
- PMCID: PMC6863159
- DOI: 10.1007/s00280-017-3438-y
Randomized study of etirinotecan pegol versus irinotecan as second-line treatment for metastatic colorectal cancer
Abstract
Purpose: Etirinotecan pegol (EP) is a long-acting topoisomerase-I inhibitor designed to provide sustained exposure to SN-38 (active metabolite of irinotecan). This phase II study compared EP versus irinotecan as second-line treatment for KRAS-mutant, irinotecan-naïve, metastatic colorectal cancer (mCRC).
Methods: Patients were randomized to EP 145 mg/m2 or irinotecan 350 mg/m2 Q21d until disease progression/unacceptable toxicity. The primary endpoint was progression-free survival (PFS) with response determined by central radiologic review (RECIST version 1.1).
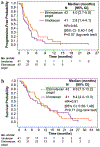
Results: The study was terminated before completing accrual due to evolving standards of care. Eighty-three patients were randomized. Median PFS was longer with EP versus irinotecan (4.0 versus 2.8 months, respectively; HR 0.65; 95% CI 0.40-1.04; P = 0.07). Six-month PFS rates were 32.8 and 15.4%, respectively. Median OS was 9.6 and 8.4 months in EP and irinotecan arms, respectively (HR 0.91; 95% CI 0.56-1.49). ORRs were 10 and 5%, respectively (P = 0.676); median DOR was significantly longer in EP arm (7.9 versus 1.4 months; P = 0.018). The most common grade-3/4 adverse events for EP and irinotecan were diarrhea (21 vs 20%), neutropenia (10 vs 22%), abdominal pain (14 vs 5%), nausea (14 vs 2%), and vomiting (12 vs 7%), respectively.
Conclusion: EP is active and safe for second-line treatment of KRAS-mutant, irinotecan-naïve mCRC.
Keywords: Chemotherapy; Etirinotecan pegol; Irinotecan; KRAS mutant; Metastatic colorectal cancer.
Conflict of interest statement
Figures
Similar articles
-
Randomized multicenter phase II trial comparing two schedules of etirinotecan pegol (NKTR-102) in women with recurrent platinum-resistant/refractory epithelial ovarian cancer.J Clin Oncol. 2013 Nov 10;31(32):4060-6. doi: 10.1200/JCO.2012.45.1278. Epub 2013 Sep 30. J Clin Oncol. 2013. PMID: 24081946 Free PMC article. Clinical Trial.
-
Etirinotecan pegol (NKTR-102) versus treatment of physician's choice in women with advanced breast cancer previously treated with an anthracycline, a taxane, and capecitabine (BEACON): a randomised, open-label, multicentre, phase 3 trial.Lancet Oncol. 2015 Nov;16(15):1556-1568. doi: 10.1016/S1470-2045(15)00332-0. Epub 2015 Oct 22. Lancet Oncol. 2015. PMID: 26482278 Clinical Trial.
-
A multicenter, open-label, expanded phase 2 study to evaluate the safety and efficacy of etirinotecan pegol, a polymer conjugate of irinotecan, in women with recurrent platinum-resistant or refractory ovarian cancer.Gynecol Oncol. 2017 Nov;147(2):276-282. doi: 10.1016/j.ygyno.2017.08.026. Epub 2017 Sep 19. Gynecol Oncol. 2017. PMID: 28935273 Clinical Trial.
-
Etirinotecan pegol for the treatment of breast cancer.Expert Opin Pharmacother. 2016;17(5):727-34. doi: 10.1517/14656566.2016.1154537. Epub 2016 Mar 10. Expert Opin Pharmacother. 2016. PMID: 26881332 Review.
-
Etirinotecan pegol: development of a novel conjugated topoisomerase I inhibitor.Curr Oncol Rep. 2014 Feb;16(2):367. doi: 10.1007/s11912-013-0367-8. Curr Oncol Rep. 2014. PMID: 24445499 Review.
Cited by
-
Overview of the New Bioactive Heterocycles as Targeting Topoisomerase Inhibitors Useful Against Colon Cancer.Anticancer Agents Med Chem. 2024;24(4):236-262. doi: 10.2174/0118715206269722231121173311. Anticancer Agents Med Chem. 2024. PMID: 38038012 Review.
-
Global Research Trends and Hotspots in the Role of Cholesterol in Colorectal Cancer: A Bibliometric Analysis.Hum Mutat. 2025 May 24;2025:6546114. doi: 10.1155/humu/6546114. eCollection 2025. Hum Mutat. 2025. PMID: 40452858 Free PMC article.
-
Second-line systemic treatment for metastatic colorectal cancer: A systematic review and Bayesian network meta-analysis based on RCT.PLoS One. 2024 Dec 23;19(12):e0313278. doi: 10.1371/journal.pone.0313278. eCollection 2024. PLoS One. 2024. PMID: 39715232 Free PMC article.
-
Effects of sevoflurane and propofol on postoperative nausea and vomiting in patients with colorectal cancer placed under general anesthesia: a systematic review and meta-analysis.J Gastrointest Oncol. 2022 Dec;13(6):2963-2972. doi: 10.21037/jgo-22-783. J Gastrointest Oncol. 2022. PMID: 36636047 Free PMC article.
-
The Validity of Surrogate Endpoints in Sub Groups of Metastatic Colorectal Cancer Patients Defined by Treatment Class and KRAS Status.Cancers (Basel). 2022 Nov 1;14(21):5391. doi: 10.3390/cancers14215391. Cancers (Basel). 2022. PMID: 36358810 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30 - PubMed
-
- Surveillance, epidemiology, and end results program: SEER stat facts sheet: colon and rectum cancer. http://seer.cancer.gov/stat-facts/html/colorect.html. Cited 8 Feb 2017
-
- Gustavsson B, Carlsson G, Machover D, Petrelli N, Roth A, Schmoll HJ et al. (2015) A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer 14:1–10 - PubMed
-
- National Comprehensive Cancer Network: clinical practice guidelines in oncology (NCCN guidelines®): colon cancer version 1.2017, 11/16 update. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Cited 8 Feb 2017
-
- Lucas AS, O’Neil BH, Goldberg RM (2011) A decade of advances in cytotoxic chemotherapy for metastatic colorectal cancer. Clin Colorectal Cancer 10:238–244 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous