Taurine as a water structure breaker and protein stabilizer
- PMID: 29043510
- PMCID: PMC5762795
- DOI: 10.1007/s00726-017-2499-x
Taurine as a water structure breaker and protein stabilizer
Abstract
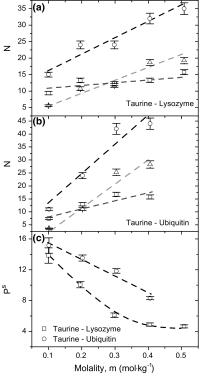
The enhancing effect on the water structure has been confirmed for most of the osmolytes exhibiting both stabilizing and destabilizing properties in regard to proteins. The presented work concerns osmolytes, which should be classified as "structure breaking" solutes: taurine and N,N,N-trimethyltaurine (TMT). Here, we combine FTIR spectroscopy, DSC calorimetry and DFT calculations to gain an insight into the interactions between osmolytes and two proteins: lysozyme and ubiquitin. Despite high structural similarity, both osmolytes exert different influence on protein stability: taurine is a stabilizer, TMT is a denaturant. We show also that taurine amino group interacts directly with the side chains of proteins, whereas TMT does not interact with proteins at all. Although two solutes weaken on average the structure of the surrounding water, their hydration spheres are different. Taurine is surrounded by two populations of water molecules: bonded with weak H-bonds around sulfonate group, and strongly bonded around amino group. The strong hydrogen-bonded network of water molecules around the amino group of taurine further improves properties of enhanced protein hydration sphere and stabilizes the native protein form. Direct interactions of this group with surface side chains provide a proper orientation of taurine and prevents the [Formula: see text] group from negative influence. The weakened [Formula: see text] hydration sphere of TMT breaks up the hydrogen-bonded network of water around the protein and destabilizes it. However, TMT at low concentration stabilize both proteins to a small extent. This effect can be attributed to an actual osmophobic effect which is overcome if the concentration increases.
Keywords: DFT calculations; DSC calorimetry; FTIR spectroscopy; Hydration; Protein interactions; Taurine.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors, thus no informed consent was required in this study.
Figures





References
-
- Barnhurst JD. Dipolar ions related to taurine. J Org Chem. 1961;26:4520–4522. doi: 10.1021/jo01069a077. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

