Comparison of Shallow and Deep Learning Methods on Classifying the Regional Pattern of Diffuse Lung Disease
- PMID: 29043528
- PMCID: PMC6113148
- DOI: 10.1007/s10278-017-0028-9
Comparison of Shallow and Deep Learning Methods on Classifying the Regional Pattern of Diffuse Lung Disease
Abstract
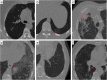
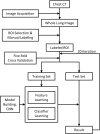
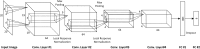
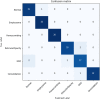
This study aimed to compare shallow and deep learning of classifying the patterns of interstitial lung diseases (ILDs). Using high-resolution computed tomography images, two experienced radiologists marked 1200 regions of interest (ROIs), in which 600 ROIs were each acquired using a GE or Siemens scanner and each group of 600 ROIs consisted of 100 ROIs for subregions that included normal and five regional pulmonary disease patterns (ground-glass opacity, consolidation, reticular opacity, emphysema, and honeycombing). We employed the convolution neural network (CNN) with six learnable layers that consisted of four convolution layers and two fully connected layers. The classification results were compared with the results classified by a shallow learning of a support vector machine (SVM). The CNN classifier showed significantly better performance for accuracy compared with that of the SVM classifier by 6-9%. As the convolution layer increases, the classification accuracy of the CNN showed better performance from 81.27 to 95.12%. Especially in the cases showing pathological ambiguity such as between normal and emphysema cases or between honeycombing and reticular opacity cases, the increment of the convolution layer greatly drops the misclassification rate between each case. Conclusively, the CNN classifier showed significantly greater accuracy than the SVM classifier, and the results implied structural characteristics that are inherent to the specific ILD patterns.
Keywords: Convolution neural network; Deep architecture; Interscanner variation; Interstitial lung disease; Support vector machine.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

