A Novel Negative Allosteric Modulator Selective for GluN2C/2D-Containing NMDA Receptors Inhibits Synaptic Transmission in Hippocampal Interneurons
- PMID: 29043770
- PMCID: PMC5924706
- DOI: 10.1021/acschemneuro.7b00329
A Novel Negative Allosteric Modulator Selective for GluN2C/2D-Containing NMDA Receptors Inhibits Synaptic Transmission in Hippocampal Interneurons
Abstract
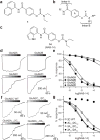
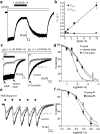
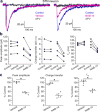
N-Methyl-d-aspartate receptors (NMDARs) are ionotropic glutamate receptors that mediate excitatory synaptic transmission and have been implicated in numerous neurological disorders. NMDARs typically comprise two GluN1 and two GluN2 subunits. The four GluN2 subtypes (GluN2A-GluN2D) have distinct functional properties and gene expression patterns, which contribute to diverse functional roles for NMDARs in the brain. Here, we present a series of GluN2C/2D-selective negative allosteric modulators built around a N-aryl benzamide (NAB) core. The prototypical compound, NAB-14, is >800-fold selective for recombinant GluN2C/GluN2D over GluN2A/GluN2B in Xenopus oocytes and has an IC50 value of 580 nM at recombinant GluN2D-containing receptors expressed in mammalian cells. NAB-14 inhibits triheteromeric (GluN1/GluN2A/GluN2C) NMDARs with modestly reduced potency and efficacy compared to diheteromeric (GluN1/GluN2C/GluN2C) receptors. Site-directed mutagenesis suggests that structural determinants for NAB-14 inhibition reside in the GluN2D M1 transmembrane helix. NAB-14 inhibits GluN2D-mediated synaptic currents in rat subthalamic neurons and mouse hippocampal interneurons, but has no effect on synaptic transmission in hippocampal pyramidal neurons, which do not express GluN2C or GluN2D. This series possesses some druglike physical properties and modest brain permeability in rat and mouse. Altogether, this work identifies a new series of negative allosteric modulators that are valuable tools for studying GluN2C- and GluN2D-containing NMDAR function in brain circuits, and suggests that the series has the potential to be developed into therapies for selectively modulating brain circuits involving the GluN2C and GluN2D subunits.
Keywords: GluN2C; GluN2D; NAB-14; NMDA receptor; NMDAR; glutamate receptor; negative allosteric modulator.
Conflict of interest statement
SFT is a consultant for Boehringer-Ingleheim Pharma GmbH and Janssen Pharmaceuticals Inc., is the principle investigator on a research grant from Janssen Pharmaceuticals Inc. to Emory University School of Medicine, is a member of the SAB for Sage Therapeuetics, and is co-founder of NeurOp Inc. DCL is a member of the Board of Directors for NeurOp Inc. DCL, SFT, SLS, DSM, TMA, SSZ, and CAM are co-inventors on Emory-owned intellectual property associated with allosteric modulators of NMDAR function.
Figures

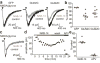

Similar articles
-
PTC-174, a positive allosteric modulator of NMDA receptors containing GluN2C or GluN2D subunits.Neuropharmacology. 2020 Aug 15;173:107971. doi: 10.1016/j.neuropharm.2020.107971. Epub 2020 Jan 25. Neuropharmacology. 2020. PMID: 31987864 Free PMC article.
-
Distinct GluN1 and GluN2 Structural Determinants for Subunit-Selective Positive Allosteric Modulation of N-Methyl-d-aspartate Receptors.ACS Chem Neurosci. 2021 Jan 6;12(1):79-98. doi: 10.1021/acschemneuro.0c00561. Epub 2020 Dec 16. ACS Chem Neurosci. 2021. PMID: 33326224 Free PMC article.
-
Functional and pharmacological properties of triheteromeric GluN1/2B/2D NMDA receptors.J Physiol. 2019 Nov;597(22):5495-5514. doi: 10.1113/JP278168. Epub 2019 Nov 2. J Physiol. 2019. PMID: 31541561 Free PMC article.
-
Investigation of the structural requirements for N-methyl-D-aspartate receptor positive and negative allosteric modulators based on 2-naphthoic acid.Eur J Med Chem. 2019 Feb 15;164:471-498. doi: 10.1016/j.ejmech.2018.12.054. Epub 2018 Dec 28. Eur J Med Chem. 2019. PMID: 30622023 Free PMC article. Review.
-
Allosteric antagonist action at triheteromeric NMDA receptors.Neuropharmacology. 2022 Jan 1;202:108861. doi: 10.1016/j.neuropharm.2021.108861. Epub 2021 Nov 2. Neuropharmacology. 2022. PMID: 34736958 Free PMC article. Review.
Cited by
-
NMDA Receptor Antagonists: Emerging Insights into Molecular Mechanisms and Clinical Applications in Neurological Disorders.Pharmaceuticals (Basel). 2024 May 15;17(5):639. doi: 10.3390/ph17050639. Pharmaceuticals (Basel). 2024. PMID: 38794209 Free PMC article. Review.
-
Di-aryl Sulfonamide Motif Adds π-Stacking Bulk in Negative Allosteric Modulators of the NMDA Receptor.ACS Med Chem Lett. 2019 Jan 4;10(3):248-254. doi: 10.1021/acsmedchemlett.8b00395. eCollection 2019 Mar 14. ACS Med Chem Lett. 2019. PMID: 30891121 Free PMC article.
-
Positive and Negative Allosteric Modulators of N-Methyl-d-aspartate (NMDA) Receptors: Structure-Activity Relationships and Mechanisms of Action.J Med Chem. 2019 Jan 10;62(1):3-23. doi: 10.1021/acs.jmedchem.7b01640. Epub 2018 Mar 5. J Med Chem. 2019. PMID: 29446949 Free PMC article. Review.
-
NMDA receptor blockade ameliorates abnormalities of spike firing of subthalamic nucleus neurons in a parkinsonian nonhuman primate.J Neurosci Res. 2018 Jul;96(7):1324-1335. doi: 10.1002/jnr.24230. Epub 2018 Mar 25. J Neurosci Res. 2018. PMID: 29577359 Free PMC article.
-
A structurally derived model of subunit-dependent NMDA receptor function.J Physiol. 2018 Sep;596(17):4057-4089. doi: 10.1113/JP276093. Epub 2018 Aug 1. J Physiol. 2018. PMID: 29917241 Free PMC article.
References
-
- Parsons MP, Raymond LA. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron. 2014;82:279–293. - PubMed
-
- Gonzalez J, Jurado-Coronel JC, Avila MF, Sabogal A, Capani F, Barreto GE. NMDARs in neurological diseases: a potential therapeutic target. Int J Neurosci 2014 - PubMed
-
- Mellone M, Gardoni F. Modulation of NMDA receptor at the synapse: promising therapeutic interventions in disorders of the nervous system. Eur J Pharmacol. 2013;719:75–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

