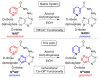
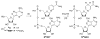Emissive Synthetic Cofactors: An Isomorphic, Isofunctional, and Responsive NAD+ Analogue
- PMID: 29043790
- PMCID: PMC5766281
- DOI: 10.1021/jacs.7b05852
Emissive Synthetic Cofactors: An Isomorphic, Isofunctional, and Responsive NAD+ Analogue
Abstract
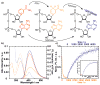
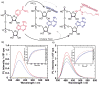
The synthesis, photophysics, and biochemical utility of a fluorescent NAD+ analogue based on an isothiazolo[4,3-d]pyrimidine core (NtzAD+) are described. Enzymatic reactions, photophysically monitored in real time, show NtzAD+ and NtzADH to be substrates for yeast alcohol dehydrogenase and lactate dehydrogenase, respectively, with reaction rates comparable to that of the native cofactors. A drop in fluorescence is seen as NtzAD+ is converted to NtzADH, reflecting a complementary photophysical behavior to that of the native NAD+/NADH. NtzAD+ and NtzADH serve as substrates for NADase, which selectively cleaves the nicotinamide's glycosidic bond yielding tzADP-ribose. NtzAD+ also serves as a substrate for ribosyl transferases, including human adenosine ribosyl transferase 5 (ART5) and Cholera toxin subunit A (CTA), which hydrolyze the nicotinamide and transfer tzADP-ribose to an arginine analogue, respectively. These reactions can be monitored by fluorescence spectroscopy, in stark contrast to the corresponding processes with the nonemissive NAD+.
Conflict of interest statement
The authors declare the following competing financial interest(s): Yitzhak Tor provides consulting services to TriLink Biotechnologies. The terms of the arrangements have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies.
Figures