Preparation and evaluation of Vinpocetine self-emulsifying pH gradient release pellets
- PMID: 29043863
- PMCID: PMC8241196
- DOI: 10.1080/10717544.2017.1388453
Preparation and evaluation of Vinpocetine self-emulsifying pH gradient release pellets
Abstract
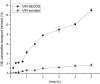
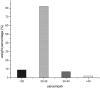
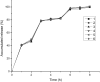
The main objective of this study was to develop a pH gradient release pellet with self-emulsifying drug delivery system (SEDDS), which could not only improve the oral bioavailability of Vinpocetine (VIN), a poor soluble drug, but reduce the fluctuation of plasma concentration. First, the liquid VIN SEDDS formulation was prepared. Then the self-emulsifying pH gradient release pellets were prepared by extrusion spheronization technique, and formulation consisted by the liquid SEDDS, absorbent (colloidal silicon dioxide), penetration enhancer (sodium chloride), microcrystalline cellulose, ethyl alcohol, and three coating materials (HPMC, Eudragit L30D55, Eudragit FS30D) were eventually selected. Three kinds of coated pellets were mixed in capsules with the mass ratio of 1:1:1. The release curves of capsules were investigated in vitro under the simulated gastrointestinal conditions. In addition, the oral bioavailability and pharmacokinetics of VIN self-emulsifying pH gradient release pellets, commercial tablets and liquid VIN SEDDS were evaluated in Beagle dogs. The oral bioavailability of self-emulsifying pH gradient release pellets was about 149.8% of commercial VIN tablets, and it was about 86% of liquid VIN SEDDS, but there were no significant difference between liquid SEDDS and self-emulsifying pH gradient release pellets. In conclusion, the self-emulsifying pH gradient release pellets could significantly enhance the absorption of VIN and effectively achieve a pH gradient release. And the self-emulsifying pH gradient release pellet was a promising method to improve bioavailability of insoluble drugs.
Keywords: Self-emulsifying drug delivery system; Vinpocetine; oral bioavailability; pH gradient pellet; poorly water-soluble drugs.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures


Similar articles
-
Solid self-emulsifying nitrendipine pellets: preparation and in vitro/in vivo evaluation.Int J Pharm. 2010 Jan 4;383(1-2):1-6. doi: 10.1016/j.ijpharm.2009.08.014. Epub 2009 Aug 19. Int J Pharm. 2010. PMID: 19698771
-
Controlled release of oral tetrahydrocurcumin from a novel self-emulsifying floating drug delivery system (SEFDDS).AAPS PharmSciTech. 2011 Mar;12(1):152-64. doi: 10.1208/s12249-010-9568-8. Epub 2010 Dec 23. AAPS PharmSciTech. 2011. PMID: 21181511 Free PMC article.
-
Bi-layered self-emulsifying pellets prepared by co-extrusion and spheronization: influence of formulation variables and preliminary study on the in vivo absorption.Eur J Pharm Biopharm. 2008 Jun;69(2):686-97. doi: 10.1016/j.ejpb.2007.11.014. Epub 2007 Dec 4. Eur J Pharm Biopharm. 2008. PMID: 18191390
-
A Comprehensive Insight on Recent Advancements in Self-emulsifying Drug Delivery Systems.Curr Drug Deliv. 2023;20(8):1095-1114. doi: 10.2174/1567201819666220914113324. Curr Drug Deliv. 2023. PMID: 36111756 Review.
-
Preparation of the traditional Chinese medicine compound recipe heart-protecting musk pH-dependent gradient-release pellets.Drug Dev Ind Pharm. 2002 Nov;28(10):1261-73. doi: 10.1081/ddc-120015360. Drug Dev Ind Pharm. 2002. PMID: 12476872 Review.
Cited by
-
Current Status of Supersaturable Self-Emulsifying Drug Delivery Systems.Pharmaceutics. 2020 Apr 16;12(4):365. doi: 10.3390/pharmaceutics12040365. Pharmaceutics. 2020. PMID: 32316199 Free PMC article. Review.
-
Solid Sirolimus Self-microemulsifying Drug Delivery System: Development and Evaluation of Tablets with Sustained Release Property.Iran J Pharm Res. 2019 Fall;18(4):1648-1658. doi: 10.22037/ijpr.2019.1100847. Iran J Pharm Res. 2019. PMID: 32184836 Free PMC article.
-
Recent Trends in Assessment of Cellulose Derivatives in Designing Novel and Nanoparticulate-Based Drug Delivery Systems for Improvement of Oral Health.Polymers (Basel). 2021 Dec 27;14(1):92. doi: 10.3390/polym14010092. Polymers (Basel). 2021. PMID: 35012115 Free PMC article. Review.
-
Patient-Friendly, Olfactory-Targeted, Stimuli-Responsive Hydrogels for Cerebral Degenerative Disorders Ensured > 400% Brain Targeting Efficiency in Rats.AAPS PharmSciTech. 2020 Nov 22;22(1):6. doi: 10.1208/s12249-020-01872-0. AAPS PharmSciTech. 2020. PMID: 33222021
-
Formulation and clinical investigation of optimized vinpocetine lyoplant-tabs: new strategy in development of buccal solid dosage form.Drug Des Devel Ther. 2018 Dec 28;13:205-220. doi: 10.2147/DDDT.S189105. eCollection 2019. Drug Des Devel Ther. 2018. PMID: 30643387 Free PMC article.
References
-
- Abdalla A, Mader K. (2007). Preparation and characterization of a self-emulsifying pellet formulation. Eur J Pharm Biopharm 66:220–6. - PubMed
-
- Agrawal U, Sharma R, Gupta M, Vyas SP. (2014). Is nanotechnology a boon for oral drug delivery? Drug Discov Today 19:1530–46. - PubMed
-
- Aho J, Halme A, Boetker J, et al. . (2017). The effect of HPMC and MC as pore formers on the rheology of the implant microenvironment and the drug release in vitro. Carbohydrate Polymers 177:433–42. - PubMed
-
- Aungst BJ. (1993). Novel formulation strategies for improving oral bioavailability of drugs with poor membrane permeation or presystemic metabolism. Cheminform 82:979–87. - PubMed
-
- Bereczki D, Fekete I. (1999). A systematic review of vinpocetine therapy in acute ischaemic stroke. Eur J Clin Pharmacol 55:349. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
