N-acetylglucosamine affects Cryptococcus neoformans cell-wall composition and melanin architecture
- PMID: 29043954
- PMCID: PMC5775898
- DOI: 10.1099/mic.0.000552
N-acetylglucosamine affects Cryptococcus neoformans cell-wall composition and melanin architecture
Abstract
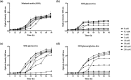
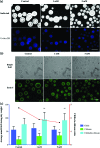
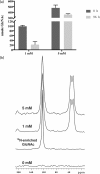
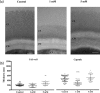
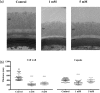
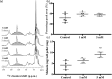
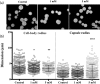
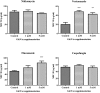
Cryptococcus neoformans is an environmental fungus that belongs to the phylum Basidiomycetes and is a major pathogen in immunocompromised patients. The ability of C. neoformans to produce melanin pigments represents its second most important virulence factor, after the presence of a polysaccharide capsule. Both the capsule and melanin are closely associated with the fungal cell wall, a complex structure that is essential for maintaining cell morphology and viability under conditions of stress. The amino sugar N-acetylglucosamine (GlcNAc) is a key constituent of the cell-wall chitin and is used for both N-linked glycosylation and GPI anchor synthesis. Recent studies have suggested additional roles for GlcNAc as an activator and mediator of cellular signalling in fungal and plant cells. Furthermore, chitin and chitosan polysaccharides interact with melanin pigments in the cell wall and have been found to be essential for melanization. Despite the importance of melanin, its molecular structure remains unresolved; however, we previously obtained critical insights using advanced nuclear magnetic resonance (NMR) and imaging techniques. In this study, we investigated the effect of GlcNAc supplementation on cryptococcal cell-wall composition and melanization. C. neoformans was able to metabolize GlcNAc as a sole source of carbon and nitrogen, indicating a capacity to use a component of a highly abundant polymer in the biospherenutritionally. C. neoformans cells grown with GlcNAc manifested changes in the chitosan cell-wall content, cell-wall thickness and capsule size. Supplementing cultures with isotopically 15N-labelled GlcNAc demonstrated that the exogenous monomer serves as a building block for chitin/chitosan and is incorporated into the cell wall. The altered chitin-to-chitosan ratio had no negative effects on the mother-daughter cell separation; growth with GlcNAc affected the fungal cell-wall scaffold, resulting in increased melanin deposition and assembly. In summary, GlcNAc supplementation had pleiotropic effects on cell-wall and melanin architectures, and thus established its capacity to perturb these structures, a property that could prove useful for metabolic tracking studies.
Keywords: N-acetylglucosamine; chitin; chitosan; fungal cell wall; melanin.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

Similar articles
-
Cell-wall dyes interfere with Cryptococcus neoformans melanin deposition.Microbiology (Reading). 2018 Aug;164(8):1012-1022. doi: 10.1099/mic.0.000682. Epub 2018 Jun 25. Microbiology (Reading). 2018. PMID: 29939127 Free PMC article.
-
Melanin deposition in two Cryptococcus species depends on cell-wall composition and flexibility.J Biol Chem. 2020 Feb 14;295(7):1815-1828. doi: 10.1074/jbc.RA119.011949. Epub 2020 Jan 2. J Biol Chem. 2020. PMID: 31896575 Free PMC article.
-
Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans.Eukaryot Cell. 2007 May;6(5):855-67. doi: 10.1128/EC.00399-06. Epub 2007 Mar 30. Eukaryot Cell. 2007. PMID: 17400891 Free PMC article.
-
Capsule and melanin synthesis in Cryptococcus neoformans.Med Mycol. 2001;39 Suppl 1:19-30. Med Mycol. 2001. PMID: 11800266 Review.
-
Synthesis and assembly of fungal melanin.Appl Microbiol Biotechnol. 2012 Feb;93(3):931-40. doi: 10.1007/s00253-011-3777-2. Epub 2011 Dec 16. Appl Microbiol Biotechnol. 2012. PMID: 22173481 Free PMC article. Review.
Cited by
-
Wor1-regulated ferroxidases contribute to pigment formation in opaque cells of Candida albicans.FEBS Open Bio. 2021 Mar;11(3):598-621. doi: 10.1002/2211-5463.13070. Epub 2021 Feb 18. FEBS Open Bio. 2021. PMID: 33350590 Free PMC article.
-
The melanization road more traveled by: Precursor substrate effects on melanin synthesis in cell-free and fungal cell systems.J Biol Chem. 2018 Dec 28;293(52):20157-20168. doi: 10.1074/jbc.RA118.005791. Epub 2018 Nov 1. J Biol Chem. 2018. PMID: 30385508 Free PMC article.
-
Cell-wall dyes interfere with Cryptococcus neoformans melanin deposition.Microbiology (Reading). 2018 Aug;164(8):1012-1022. doi: 10.1099/mic.0.000682. Epub 2018 Jun 25. Microbiology (Reading). 2018. PMID: 29939127 Free PMC article.
-
N-Acetylglucosamine Regulates Morphogenesis and Virulence Pathways in Fungi.J Fungi (Basel). 2019 Dec 24;6(1):8. doi: 10.3390/jof6010008. J Fungi (Basel). 2019. PMID: 31878148 Free PMC article. Review.
-
Molecular Mechanisms of Chitosan Interactions with Fungi and Plants.Int J Mol Sci. 2019 Jan 15;20(2):332. doi: 10.3390/ijms20020332. Int J Mol Sci. 2019. PMID: 30650540 Free PMC article. Review.
References
-
- Casadevall A, Perfect JR. Cryptococcus Neoformans. Washington, DC: American Society for Microbiology; 1998.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

