Myosin V: Chemomechanical-coupling ratchet with load-induced mechanical slip
- PMID: 29044145
- PMCID: PMC5647391
- DOI: 10.1038/s41598-017-13661-0
Myosin V: Chemomechanical-coupling ratchet with load-induced mechanical slip
Abstract
A chemomechanical-network model for myosin V is presented on the basis of both the nucleotide-dependent binding affinity of the head to an actin filament (AF) and asymmetries and similarity relations among the chemical transitions due to an intramolecular strain of the leading and trailing heads. The model allows for branched chemomechanical cycles and takes into account not only two different force-generating mechanical transitions between states wherein the leading head is strongly bound and the trailing head is weakly bound to the AF but also load-induced mechanical-slip transitions between states in which both heads are strongly bound. The latter is supported by the fact that ATP-independent high-speed backward stepping has been observed for myosin V, although such motility has never been for kinesin. The network model appears as follows: (1) the high chemomechanical-coupling ratio between forward step and ATP hydrolysis is achieved even at low ATP concentrations by the dual mechanical transitions; (2) the forward stepping at high ATP concentrations is explained by the front head-gating mechanism wherein the power stroke is triggered by the inorganic-phosphate (Pi) release from the leading head; (3) the ATP-binding or hydrolyzed ADP.Pi-binding leading head produces a stable binding to the AF, especially against backward loading.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
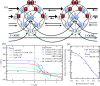


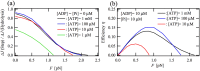

 and
and  indicate the forward- and backward-stepping transitions, respectively. The red numbers indicate the probability of the state. The perpendicular axis for each diagram indicates the ATP-concentration level; higher states on this axis have higher probabilities at high ATP concentrations.
indicate the forward- and backward-stepping transitions, respectively. The red numbers indicate the probability of the state. The perpendicular axis for each diagram indicates the ATP-concentration level; higher states on this axis have higher probabilities at high ATP concentrations.Similar articles
-
Design principles governing chemomechanical coupling of kinesin.Sci Rep. 2017 Apr 25;7(1):1163. doi: 10.1038/s41598-017-01328-9. Sci Rep. 2017. PMID: 28442770 Free PMC article.
-
An ionic-chemical-mechanical model for muscle contraction.Biopolymers. 2016 Dec;105(12):887-97. doi: 10.1002/bip.22926. Biopolymers. 2016. PMID: 27603027
-
Chemomechanical cycle of kinesin differs from that of myosin.Nature. 1993 Jan 14;361(6408):168-70. doi: 10.1038/361168a0. Nature. 1993. PMID: 8421522
-
Actin polymerization: regulation by divalent metal ion and nucleotide binding, ATP hydrolysis and binding of myosin.Adv Exp Med Biol. 1994;358:71-81. doi: 10.1007/978-1-4615-2578-3_7. Adv Exp Med Biol. 1994. PMID: 7801813 Review.
-
Electron Microscopic Recording of the Power and Recovery Strokes of Individual Myosin Heads Coupled with ATP Hydrolysis: Facts and Implications.Int J Mol Sci. 2018 May 4;19(5):1368. doi: 10.3390/ijms19051368. Int J Mol Sci. 2018. PMID: 29734671 Free PMC article. Review.
Cited by
-
Myosin V executes steps of variable length via structurally constrained diffusion.Elife. 2020 Jan 15;9:e51569. doi: 10.7554/eLife.51569. Elife. 2020. PMID: 31939739 Free PMC article.
-
Mechanism underlying hippocampal long-term potentiation and depression based on competition between endocytosis and exocytosis of AMPA receptors.Sci Rep. 2020 Sep 7;10(1):14711. doi: 10.1038/s41598-020-71528-3. Sci Rep. 2020. PMID: 32895399 Free PMC article.
-
Multiscale kinetic analysis of proteins.Curr Opin Struct Biol. 2022 Feb;72:169-175. doi: 10.1016/j.sbi.2021.11.005. Epub 2021 Dec 16. Curr Opin Struct Biol. 2022. PMID: 34923310 Free PMC article. Review.
-
Muscarinic acetylcholine receptor-dependent and NMDA receptor-dependent LTP and LTD share the common AMPAR trafficking pathway.iScience. 2023 Feb 3;26(3):106133. doi: 10.1016/j.isci.2023.106133. eCollection 2023 Mar 17. iScience. 2023. PMID: 36866246 Free PMC article.
-
Multiscale Responsive Kinetic Modeling: Quantifying Biomolecular Reaction Flux under Varying Electrochemical Conditions.bioRxiv [Preprint]. 2024 Aug 2:2024.08.01.606205. doi: 10.1101/2024.08.01.606205. bioRxiv. 2024. Update in: J Chem Theory Comput. 2025 Jan 14;21(1):374-389. doi: 10.1021/acs.jctc.4c00872. PMID: 39131358 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

