Wax Ester Synthase/Diacylglycerol Acyltransferase Isoenzymes Play a Pivotal Role in Wax Ester Biosynthesis in Euglena gracilis
- PMID: 29044218
- PMCID: PMC5647427
- DOI: 10.1038/s41598-017-14077-6
Wax Ester Synthase/Diacylglycerol Acyltransferase Isoenzymes Play a Pivotal Role in Wax Ester Biosynthesis in Euglena gracilis
Abstract
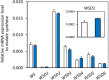
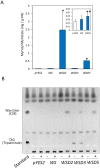
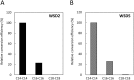
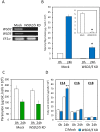
Wax ester fermentation is a unique energy gaining pathway for a unicellular phytoflagellated protozoan, Euglena gracilis, to survive under anaerobiosis. Wax esters produced in E. gracilis are composed of saturated fatty acids and alcohols, which are the major constituents of myristic acid and myristyl alcohol. Thus, wax esters can be promising alternative biofuels. Here, we report the identification and characterization of wax ester synthase/diacylglycerol acyltrasferase (WSD) isoenzymes as the terminal enzymes of wax ester production in E. gracilis. Among six possible Euglena WSD orthologs predicted by BLASTX search, gene expression analysis and in vivo evaluation for enzyme activity with yeast expressing individual recombinant WSDs indicated that two of them (EgWSD2 and EgWSD5) predominantly function as wax ester synthase. Furthermore, experiments with gene silencing demonstrated a pivotal role of both EgWSD2 and EgWSD5 in wax ester synthesis, as evidenced by remarkably reduced wax ester contents in EgWSD2/5-double knockdown E. gracilis cells treated with anaerobic conditions. Interestingly, the decreased ability to produce wax ester did not affect adaptation of E. gracilis to anaerobiosis. Lipid profile analysis suggested allocation of metabolites to other compounds including triacylglycerol instead of wax esters.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Fatty acyl-CoA reductase and wax synthase from Euglena gracilis in the biosynthesis of medium-chain wax esters.Lipids. 2010 Mar;45(3):263-73. doi: 10.1007/s11745-010-3395-2. Epub 2010 Mar 2. Lipids. 2010. PMID: 20195781
-
Alteration of Wax Ester Content and Composition in Euglena gracilis with Gene Silencing of 3-ketoacyl-CoA Thiolase Isozymes.Lipids. 2015 May;50(5):483-92. doi: 10.1007/s11745-015-4010-3. Epub 2015 Apr 10. Lipids. 2015. PMID: 25860691
-
Wax Ester Fermentation and Its Application for Biofuel Production.Adv Exp Med Biol. 2017;979:269-283. doi: 10.1007/978-3-319-54910-1_13. Adv Exp Med Biol. 2017. PMID: 28429326 Review.
-
Variability of wax ester fermentation in natural and bleached Euglena gracilis Strains in response to oxygen and the elongase inhibitor flufenacet.J Eukaryot Microbiol. 2010 Jan-Feb;57(1):63-9. doi: 10.1111/j.1550-7408.2009.00452.x. Epub 2009 Dec 10. J Eukaryot Microbiol. 2010. PMID: 20015184
-
Understanding wax ester synthesis in Euglena gracilis: Insights into mitochondrial anaerobic respiration.Protist. 2023 Dec;174(6):125996. doi: 10.1016/j.protis.2023.125996. Epub 2023 Nov 21. Protist. 2023. PMID: 38041972 Review.
Cited by
-
Fatty Acids Derivatives From Eukaryotic Microalgae, Pathways and Potential Applications.Front Microbiol. 2021 Sep 29;12:718933. doi: 10.3389/fmicb.2021.718933. eCollection 2021. Front Microbiol. 2021. PMID: 34659147 Free PMC article. Review.
-
Bioproducts From Euglena gracilis: Synthesis and Applications.Front Bioeng Biotechnol. 2019 May 15;7:108. doi: 10.3389/fbioe.2019.00108. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31157220 Free PMC article. Review.
-
Increase in alpha-linolenic acid content by simultaneous expression of fatty acid metabolism genes in Sesame (Sesamum indicum L.).Physiol Mol Biol Plants. 2022 Mar;28(3):559-572. doi: 10.1007/s12298-022-01152-0. Epub 2022 Mar 22. Physiol Mol Biol Plants. 2022. PMID: 35465201 Free PMC article.
-
A major isoform of mitochondrial trans-2-enoyl-CoA reductase is dispensable for wax ester production in Euglena gracilis under anaerobic conditions.PLoS One. 2019 Jan 16;14(1):e0210755. doi: 10.1371/journal.pone.0210755. eCollection 2019. PLoS One. 2019. PMID: 30650145 Free PMC article.
-
Cuticular wax in wheat: biosynthesis, genetics, and the stress response.Front Plant Sci. 2024 Dec 3;15:1498505. doi: 10.3389/fpls.2024.1498505. eCollection 2024. Front Plant Sci. 2024. PMID: 39703555 Free PMC article. Review.
References
-
- Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010;14:217–232. doi: 10.1016/j.rser.2009.07.020. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

