Disease and Region Specificity of Granulin Immunopositivities in Alzheimer Disease and Frontotemporal Lobar Degeneration
- PMID: 29044416
- PMCID: PMC5939662
- DOI: 10.1093/jnen/nlx085
Disease and Region Specificity of Granulin Immunopositivities in Alzheimer Disease and Frontotemporal Lobar Degeneration
Abstract
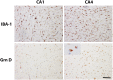
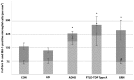
Heterozygous loss-of-function mutations in GRN, the progranulin gene, which result in progranulin (PGRN) protein haploinsufficiency, are a major cause of frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP). PGRN is composed of seven and a half repeats of a highly conserved granulin motif that is cleaved to produce the granulin peptides A-G and paragranulin. To better understand the role of PGRN and granulin (Grn) peptides in the pathogenesis of neurodegeneration, we evaluated PGRN/Grn in brains of patients with Alzheimer disease, FTLD-TDP type A with or without GRN mutations, and normal individuals, using a panel of monoclonal antibodies against Grn peptides A-G. In the neocortex, Grn peptide-specific immunostains were observed, for example, membranous Grn E immunopositivity in pyramidal neurons, and Grn C immunopositivity in ramified microglia. In the hippocampus, Grn immunopositivity in the CA1 and CA2 regions showed disease-specific changes in both neurons and microglia. Most interestingly, in FTLD-TDP type A with GRN mutations, there is a 60% decrease in the density of Grn-positive microglia in the hippocampal CA1, suggesting that haploinsufficiency of the GRN mutations also extends to PGRN expression in microglia. This study provides important insights into future studies of the pathogenesis and treatment of FTLD-TDP.
Keywords: Alzheimer disease; Frontotemporal lobar degeneration; Granulin; Hippocampal sclerosis; Microglia; Neuroinflammation; Progranulin.
© 2017 American Association of Neuropathologists, Inc. All rights reserved.
Figures





Similar articles
-
FTLD-TDP With and Without GRN Mutations Cause Different Patterns of CA1 Pathology.J Neuropathol Exp Neurol. 2019 Sep 1;78(9):844-853. doi: 10.1093/jnen/nlz059. J Neuropathol Exp Neurol. 2019. PMID: 31361008 Free PMC article.
-
Progranulin deficiency induces overactivation of WNT5A expression via TNF-α/NF-κB pathway in peripheral cells from frontotemporal dementia-linked granulin mutation carriers.J Psychiatry Neurosci. 2016 Jun;41(4):225-39. doi: 10.1503/jpn.150131. J Psychiatry Neurosci. 2016. PMID: 26624524 Free PMC article.
-
Early lysosomal maturation deficits in microglia triggers enhanced lysosomal activity in other brain cells of progranulin knockout mice.Mol Neurodegener. 2018 Sep 4;13(1):48. doi: 10.1186/s13024-018-0281-5. Mol Neurodegener. 2018. PMID: 30180904 Free PMC article.
-
Progranulin and TDP-43: mechanistic links and future directions.J Mol Neurosci. 2011 Nov;45(3):561-73. doi: 10.1007/s12031-011-9625-0. Epub 2011 Aug 24. J Mol Neurosci. 2011. PMID: 21863317 Free PMC article. Review.
-
Progranulin: a new avenue towards the understanding and treatment of neurodegenerative disease.Brain. 2017 Dec 1;140(12):3081-3104. doi: 10.1093/brain/awx198. Brain. 2017. PMID: 29053785 Review.
Cited by
-
Lycium barbarum polysaccharide alleviates dextran sodium sulfate-induced inflammatory bowel disease by regulating M1/M2 macrophage polarization via the STAT1 and STAT6 pathways.Front Pharmacol. 2023 Apr 18;14:1044576. doi: 10.3389/fphar.2023.1044576. eCollection 2023. Front Pharmacol. 2023. PMID: 37144216 Free PMC article.
-
FAM76B regulates PI3K/Akt/NF-κB-mediated M1 macrophage polarization by influencing the stability of PIK3CD mRNA.Cell Mol Life Sci. 2024 Feb 29;81(1):107. doi: 10.1007/s00018-024-05133-2. Cell Mol Life Sci. 2024. PMID: 38421448 Free PMC article.
-
Differential neuropathology and functional outcome after equivalent traumatic brain injury in aged versus young adult mice.Exp Neurol. 2021 Jul;341:113714. doi: 10.1016/j.expneurol.2021.113714. Epub 2021 Apr 5. Exp Neurol. 2021. PMID: 33831399 Free PMC article.
-
Lysosomal Dysfunction and Other Pathomechanisms in FTLD: Evidence from Progranulin Genetics and Biology.Adv Exp Med Biol. 2021;1281:219-242. doi: 10.1007/978-3-030-51140-1_14. Adv Exp Med Biol. 2021. PMID: 33433878 Free PMC article.
-
Cortical and subcortical pathological burden and neuronal loss in an autopsy series of FTLD-TDP-type C.Brain. 2022 Apr 29;145(3):1069-1078. doi: 10.1093/brain/awab368. Brain. 2022. PMID: 34919645 Free PMC article.
References
-
- Ratnavalli E, Brayne C, Dawson K, et al.The prevalence of frontotemporal dementia. Neurology 2002;58:1615–21 - PubMed
-
- Arai T, Hasegawa M, Akiyama H, et al.TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 2006;351:602–11 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

