The human PKP2/plakophilin-2 gene is induced by Wnt/β-catenin in normal and colon cancer-associated fibroblasts
- PMID: 29044515
- PMCID: PMC5765413
- DOI: 10.1002/ijc.31104
The human PKP2/plakophilin-2 gene is induced by Wnt/β-catenin in normal and colon cancer-associated fibroblasts
Abstract
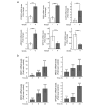
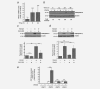
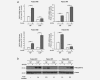
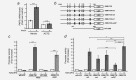
Colorectal cancer results from the malignant transformation of colonic epithelial cells. Stromal fibroblasts are the main component of the tumour microenvironment, and play an important role in the progression of this and other neoplasias. Wnt/β-catenin signalling is essential for colon homeostasis, but aberrant, constitutive activation of this pathway is a hallmark of colorectal cancer. Here we present the first transcriptomic study on the effect of a Wnt factor on human colonic myofibroblasts. Wnt3A regulates the expression of 1,136 genes, of which 662 are upregulated and 474 are downregulated in CCD-18Co cells. A set of genes encoding inhibitors of the Wnt/β-catenin pathway stand out among those induced by Wnt3A, which suggests that there is a feedback inhibitory mechanism. We also show that the PKP2 gene encoding the desmosomal protein Plakophilin-2 is a novel direct transcriptional target of Wnt/β-catenin in normal and colon cancer-associated fibroblasts. PKP2 is induced by β-catenin/TCF through three binding sites in the gene promoter and one additional binding site located in an enhancer 20 kb upstream from the transcription start site. Moreover, Plakophilin-2 antagonizes Wnt/β-catenin transcriptional activity in HEK-293T cells, which suggests that it may act as an intracellular inhibitor of the Wnt/β-catenin pathway. Our results demonstrate that stromal fibroblasts respond to canonical Wnt signalling and that Plakophilin-2 plays a role in the feedback control of this effect suggesting that the response to Wnt factors in the stroma may modulate Wnt activity in the tumour cells.
Keywords: PKP2/Plakophilin-2; Wnt/β-catenin signalling; colon cancer; gene regulation; normal and cancer-associated fibroblasts.
© 2017 The Authors International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Figures
Similar articles
-
Vitamin D and Wnt3A have additive and partially overlapping modulatory effects on gene expression and phenotype in human colon fibroblasts.Sci Rep. 2019 May 30;9(1):8085. doi: 10.1038/s41598-019-44574-9. Sci Rep. 2019. PMID: 31147591 Free PMC article.
-
Positive feedback regulation between phospholipase D and Wnt signaling promotes Wnt-driven anchorage-independent growth of colorectal cancer cells.PLoS One. 2010 Aug 12;5(8):e12109. doi: 10.1371/journal.pone.0012109. PLoS One. 2010. PMID: 20711340 Free PMC article.
-
Activation of the canonical WNT signaling pathway promotes ovarian surface epithelial proliferation without inducing β-catenin/Tcf-mediated reporter expression.Dev Dyn. 2013 Mar;242(3):291-300. doi: 10.1002/dvdy.23919. Epub 2013 Feb 6. Dev Dyn. 2013. PMID: 23239518
-
Transcriptional Regulation of Wnt/β-Catenin Pathway in Colorectal Cancer.Cells. 2020 Sep 19;9(9):2125. doi: 10.3390/cells9092125. Cells. 2020. PMID: 32961708 Free PMC article. Review.
-
Revving the Throttle on an oncogene: CDK8 takes the driver seat.Cancer Res. 2009 Oct 15;69(20):7899-901. doi: 10.1158/0008-5472.CAN-09-1704. Epub 2009 Oct 6. Cancer Res. 2009. PMID: 19808961 Free PMC article. Review.
Cited by
-
Tetrandrine inhibits proliferation of colon cancer cells by BMP9/ PTEN/ PI3K/AKT signaling.Genes Dis. 2019 Nov 8;8(3):373-383. doi: 10.1016/j.gendis.2019.10.017. eCollection 2021 May. Genes Dis. 2019. PMID: 33997184 Free PMC article.
-
Plakophilin-2 Promotes Lung Adenocarcinoma Development via Enhancing Focal Adhesion and Epithelial-Mesenchymal Transition.Cancer Manag Res. 2021 Jan 22;13:559-570. doi: 10.2147/CMAR.S281663. eCollection 2021. Cancer Manag Res. 2021. PMID: 33519235 Free PMC article.
-
Identification of PKP 2/3 as potential biomarkers of ovarian cancer based on bioinformatics and experiments.Cancer Cell Int. 2020 Oct 17;20:509. doi: 10.1186/s12935-020-01602-3. eCollection 2020. Cancer Cell Int. 2020. PMID: 33088217 Free PMC article.
-
Vitamin D Effects on Cell Differentiation and Stemness in Cancer.Cancers (Basel). 2020 Aug 25;12(9):2413. doi: 10.3390/cancers12092413. Cancers (Basel). 2020. PMID: 32854355 Free PMC article. Review.
-
The functional roles of the circRNA/Wnt axis in cancer.Mol Cancer. 2022 May 5;21(1):108. doi: 10.1186/s12943-022-01582-0. Mol Cancer. 2022. PMID: 35513849 Free PMC article. Review.
References
-
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics. CA Cancer J Clin 2015; 65:87–108. - PubMed
-
- Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomarkers Prev 2016; 25:16–27. - PubMed
-
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990; 61:759–67. - PubMed
-
- Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol 2011;6:479–507. - PubMed
-
- Clevers H, Nusse R. Wnt/β‐catenin signaling and disease. Cell 2012; 149:1192–205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

