Primary systemic therapy in resectable pancreatic ductal adenocarcinoma using mFOLFIRINOX: A pilot study
- PMID: 29044544
- PMCID: PMC5890423
- DOI: 10.1002/jso.24872
Primary systemic therapy in resectable pancreatic ductal adenocarcinoma using mFOLFIRINOX: A pilot study
Abstract
Background and objectives: Surgery followed by gemcitabine and/or a fluoropyrimidine is standard therapy for resectable PDAC. mFOLFIRINOX (oxaliplatin 85 mg/m2 , irinotecan 180 mg/m2 , leucovorin 400 mg/m2 Day 1, 5-FU 2400 mg/m2 × 48 h IV, peg-filgrastim 6 mg SQ day 3, every 14 days) has substantial activity in metastatic PDAC. We wished to determine the tolerability/efficacy of peri-operative mFOLFIRINOX in resectable PDAC.
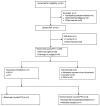
Methods: Patients with resectable PDAC (ECOG PS 0/1) received four cycles of mFOLFIRINOX pre- and post-surgery. The primary endpoint was completion of preoperative chemotherapy plus resection. Secondary endpoints included completion of all therapy, R0 resection, treatment related toxicity, PFS, and OS.
Results: Twenty-one patients enrolled: median age 62 (47-78); 20/21 (95%) completed four cycles of preoperative mFOLFIRINOX; response by RECIST was 1 CR, 3 PR, 16 SD; 17/21 (81%) completed resection, 16/21 (76%) R0; 14/21 (66%) completed four cycles of postoperative mFOLFIRINOX. Grade 3 and 4 toxicity occurred in 23% and 14% patients pre-operatively, 26% and 6.0% post-operatively. Nine patients are alive with median follow-up of 27.7 (3.1-47.1) months.
Conclusions: PST using mFOLFIRINOX in resectable PDAC is feasible and tolerable. R0 resection rate is high and survival promising, requiring longer follow-up and larger studies for definitive assessment.
Keywords: FOLFIRINOX; cancer; chemotherapy; pancreatic; resectable.
© 2017 Wiley Periodicals, Inc.
Figures
Similar articles
-
Efficacy of Preoperative mFOLFIRINOX vs mFOLFIRINOX Plus Hypofractionated Radiotherapy for Borderline Resectable Adenocarcinoma of the Pancreas: The A021501 Phase 2 Randomized Clinical Trial.JAMA Oncol. 2022 Sep 1;8(9):1263-1270. doi: 10.1001/jamaoncol.2022.2319. JAMA Oncol. 2022. PMID: 35834226 Free PMC article. Clinical Trial.
-
Safety and Efficacy of Modified FOLFIRINOX for Advanced Pancreatic Adenocarcinoma: A UK Single-Centre Experience.Oncology. 2015;89(5):281-7. doi: 10.1159/000439171. Epub 2015 Sep 16. Oncology. 2015. PMID: 26372905
-
Combining CD40 agonist mitazalimab with mFOLFIRINOX in previously untreated metastatic pancreatic ductal adenocarcinoma (OPTIMIZE-1): a single-arm, multicentre phase 1b/2 study.Lancet Oncol. 2024 Jul;25(7):853-864. doi: 10.1016/S1470-2045(24)00263-8. Epub 2024 Jun 1. Lancet Oncol. 2024. PMID: 38834087 Clinical Trial.
-
FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies.Pancreas. 2015 May;44(4):515-21. doi: 10.1097/MPA.0000000000000314. Pancreas. 2015. PMID: 25872127 Review.
-
Clinical Trials of Systemic Chemotherapy for Resectable Pancreatic Cancer: A Review.JAMA Surg. 2021 Jul 1;156(7):663-672. doi: 10.1001/jamasurg.2021.0149. JAMA Surg. 2021. PMID: 33787841 Review.
Cited by
-
Stereotactic Body Radiotherapy as an Effective Treatment for Pancreatic Cancer.Cureus. 2023 Apr 28;15(4):e38255. doi: 10.7759/cureus.38255. eCollection 2023 Apr. Cureus. 2023. PMID: 37252548 Free PMC article.
-
Clinical outcomes of FOLFIRINOX in locally advanced pancreatic cancer: A single center experience.Medicine (Baltimore). 2018 Dec;97(50):e13592. doi: 10.1097/MD.0000000000013592. Medicine (Baltimore). 2018. PMID: 30558029 Free PMC article.
-
Neoadjuvant Treatment in Patients With Resectable and Borderline Resectable Pancreatic Cancer.Front Oncol. 2020 Jan 31;10:41. doi: 10.3389/fonc.2020.00041. eCollection 2020. Front Oncol. 2020. PMID: 32083002 Free PMC article.
-
Perioperative Gemcitabine + Erlotinib Plus Pancreaticoduodenectomy for Resectable Pancreatic Adenocarcinoma: ACOSOG Z5041 (Alliance) Phase II Trial.Ann Surg Oncol. 2019 Dec;26(13):4489-4497. doi: 10.1245/s10434-019-07685-1. Epub 2019 Aug 15. Ann Surg Oncol. 2019. PMID: 31418130 Free PMC article. Clinical Trial.
-
The Emerging Role of Cyclin-Dependent Kinases (CDKs) in Pancreatic Ductal Adenocarcinoma.Int J Mol Sci. 2018 Oct 18;19(10):3219. doi: 10.3390/ijms19103219. Int J Mol Sci. 2018. PMID: 30340359 Free PMC article. Review.
References
-
- Siegel R, Miller K, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. - PubMed
-
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013. Bethesda, MD: National Cancer Institute;
-
- Ahmed SI, Bochkarev V, Oleynikov D, Sasson AR. Patients with pancreatic adenocarcinoma benefit from staging laparoscopy. J Laparoendosc Adv Surg Tech A. 2006;16:458–463. - PubMed
-
- Shah D, Fisher WE, Hodges SE, et al. Preoperative prediction of complete resection in pancreatic cancer. J Surg Res. 2008;147:216–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials