Discovery and characterization of novel trans-spliced products of human polyoma JC virus late transcripts from PML patients
- PMID: 29044559
- PMCID: PMC5805571
- DOI: 10.1002/jcp.26219
Discovery and characterization of novel trans-spliced products of human polyoma JC virus late transcripts from PML patients
Abstract
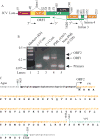
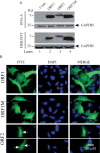
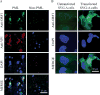
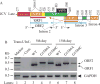
Although the human neurotropic polyomavirus, JC virus (JCV), was isolated almost a half century ago, understanding the molecular mechanisms governing its biology remains highly elusive. JCV infects oligodendrocytes and astrocytes in the central nervous system (CNS) and causes a rare fatal brain disease known as progressive multifocal leukoencephalopathy (PML) in immunocompromised individuals including AIDS. It has a small circular DNA genome (∼5 kb) and generates two primary transcripts from its early and late coding regions, producing several predicted alternatively spliced products mainly by cis-splicing. Here, we report the discovery and characterization of two novel open reading frames (ORF1 and ORF2) associated with JCV late transcripts, generated by an unusual splicing process called trans-splicing. These ORFs result from (i) the trans-splicing of two different lengths of the 5'-short coding region of VP1 between the coding regions of agnoprotein and VP2 after replacing the intron located between these two coding regions and (ii) frame-shifts occurring within the VP2 coding sequences terminated by a stop codon. ORF1 and ORF2 are capable of encoding 58 and 72 aa long proteins respectively and are expressed in infected cells and PML patients. Each ORF protein shares a common coding region with VP1 and has a unique coding sequence of their own. When the expression of the unique coding regions of ORFs is blocked by a stop codon insertion in the viral background, the mutant virus replicates less efficiently when compared to wild-type, suggesting that the newly discovered ORFs play critical roles in the JCV life cycle.
Keywords: BK virus; DNA replication; JC virus; ORF; RNA splicing; SV40; VP1; VP2; cis- and trans-splicing; merkel cell carcinoma virus; polyomavirus; progressive multifocal leukoencephalopathy; transcription.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
Authors declare no conflict of interest.
Figures







Similar articles
-
Expression of novel proteins by polyomaviruses and recent advances in the structural and functional features of agnoprotein of JC virus, BK virus, and simian virus 40.J Cell Physiol. 2019 Jun;234(6):8295-8315. doi: 10.1002/jcp.27715. Epub 2018 Nov 2. J Cell Physiol. 2019. PMID: 30390301 Free PMC article. Review.
-
Human neurotropic polyomavirus, JC virus, late coding region encodes a novel nuclear protein, ORF4, which targets the promyelocytic leukemia nuclear bodies (PML-NBs) and modulates their reorganization.Virology. 2023 Oct;587:109866. doi: 10.1016/j.virol.2023.109866. Epub 2023 Aug 19. Virology. 2023. PMID: 37741199 Free PMC article.
-
Rearranged JC virus noncoding control regions found in progressive multifocal leukoencephalopathy patient samples increase virus early gene expression and replication rate.J Virol. 2010 Oct;84(20):10448-56. doi: 10.1128/JVI.00614-10. Epub 2010 Aug 4. J Virol. 2010. PMID: 20686041 Free PMC article.
-
JC Polyomavirus Abundance and Distribution in Progressive Multifocal Leukoencephalopathy (PML) Brain Tissue Implicates Myelin Sheath in Intracerebral Dissemination of Infection.PLoS One. 2016 May 18;11(5):e0155897. doi: 10.1371/journal.pone.0155897. eCollection 2016. PLoS One. 2016. PMID: 27191595 Free PMC article.
-
An overview: Human polyomavirus JC virus and its associated disorders.J Neurovirol. 2003;9 Suppl 1:3-9. doi: 10.1080/13550280390195360. J Neurovirol. 2003. PMID: 12709864 Review.
Cited by
-
JCPyV miR-J1-5p in Urine of Natalizumab-Treated Multiple Sclerosis Patients.Viruses. 2021 Mar 12;13(3):468. doi: 10.3390/v13030468. Viruses. 2021. PMID: 33809082 Free PMC article.
-
Expression of novel proteins by polyomaviruses and recent advances in the structural and functional features of agnoprotein of JC virus, BK virus, and simian virus 40.J Cell Physiol. 2019 Jun;234(6):8295-8315. doi: 10.1002/jcp.27715. Epub 2018 Nov 2. J Cell Physiol. 2019. PMID: 30390301 Free PMC article. Review.
-
COS-7-based model: methodological approach to study John Cunningham virus replication cycle.Virol J. 2018 Feb 5;15(1):29. doi: 10.1186/s12985-018-0939-1. Virol J. 2018. PMID: 29402297 Free PMC article.
-
Human neurotropic polyomavirus, JC virus, agnoprotein targets mitochondrion and modulates its functions.Virology. 2021 Jan 15;553:135-153. doi: 10.1016/j.virol.2020.11.004. Epub 2020 Nov 25. Virology. 2021. PMID: 33278736 Free PMC article.
-
A novel chimeric RNA originating from BmCPV S4 and Bombyx mori HDAC11 transcripts regulates virus proliferation.PLoS Pathog. 2023 Dec 4;19(12):e1011184. doi: 10.1371/journal.ppat.1011184. eCollection 2023 Dec. PLoS Pathog. 2023. PMID: 38048361 Free PMC article.
References
-
- Almqvist PM, Mah R, Lendahl U, Jacobsson B, Hendson G. Immunohistochemical detection of nestin in pediatric brain tumors. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2002;50(2):147–158. - PubMed
-
- Berger JR. PML in AIDS. Neurology. 1992;42(9):1845–1846. - PubMed
-
- Berger JR. Progressive Multifocal Leukoencephalopathy. Curr Treat Options Neurol. 2000;2(4):361–368. - PubMed
-
- Berger JR. Progressive multifocal leukoencephalopathy. Curr Neurol Neurosci Rep. 2007;7(6):461–469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

