Nutrient sensing in pancreatic islets: lessons from congenital hyperinsulinism and monogenic diabetes
- PMID: 29044608
- PMCID: PMC5788728
- DOI: 10.1111/nyas.13448
Nutrient sensing in pancreatic islets: lessons from congenital hyperinsulinism and monogenic diabetes
Abstract
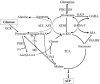
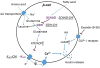
Pancreatic beta cells sense changes in nutrients during the cycles of fasting and feeding and release insulin accordingly to maintain glucose homeostasis. Abnormal beta cell nutrient sensing resulting from gene mutations leads to hypoglycemia or diabetes. Glucokinase (GCK) plays a key role in beta cell glucose sensing. As one form of congenital hyperinsulinism (CHI), activating mutations of GCK result in a decreased threshold for glucose-stimulated insulin secretion and hypoglycemia. In contrast, inactivating mutations of GCK result in diabetes, including a mild form (MODY2) and a severe form (permanent neonatal diabetes mellitus (PNDM)). Mutations of beta cell ion channels involved in insulin secretion regulation also alter glucose sensing. Activating or inactivating mutations of ATP-dependent potassium (KATP ) channel genes result in severe but completely opposite clinical phenotypes, including PNDM and CHI. Mutations of the other ion channels, including voltage-gated potassium channels (Kv 7.1) and voltage-gated calcium channels, also lead to abnormal glucose sensing and CHI. Furthermore, amino acids can stimulate insulin secretion in a glucose-independent manner in some forms of CHI, including activating mutations of the glutamate dehydrogenase gene, HDAH deficiency, and inactivating mutations of KATP channel genes. These genetic defects have provided insight into a better understanding of the complicated nature of beta cell fuel-sensing mechanisms.
Keywords: beta cell; congenital hyperinsulinism; insulin secretion; monogenic diabetes.
© 2017 New York Academy of Sciences.
Figures




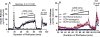
References
-
- Herchuelz A, Lebrun P, Malaisse WJ. Calcium fluxes in the process of glucose-induced insulin release. Arch Int Pharmacodyn Ther. 1980;246:173–174. - PubMed
-
- Herchuelz A, Thonnart N, Carpinelli A, et al. Regulation of calcium fluxes in rat pancreatic islets: the role of K+ conductance. J Pharmacol Exp Ther. 1980;215:213–220. - PubMed
-
- Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell metabolism. 2013;18:162–185. - PubMed
-
- Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. - PubMed
-
- Li C, Buettger C, Kwagh J, et al. A signaling role of glutamine in insulin secretion. The Journal of biological chemistry. 2004;279:13393–13401. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

