Neuroprotective Effects of Human Mesenchymal Stem Cells and Platelet-Derived Growth Factor on Human Retinal Ganglion Cells
- PMID: 29044808
- PMCID: PMC5765520
- DOI: 10.1002/stem.2722
Neuroprotective Effects of Human Mesenchymal Stem Cells and Platelet-Derived Growth Factor on Human Retinal Ganglion Cells
Abstract
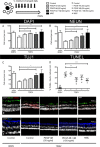
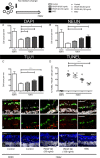
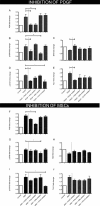
Optic neuropathies such as glaucoma occur when retinal ganglion cells (RGCs) in the eye are injured. Strong evidence suggests mesenchymal stem cells (MSCs) could be a potential therapy to protect RGCs; however, little is known regarding their effect on the human retina. We, therefore, investigated if human MSCs (hMSCs), or platelet-derived growth factor (PDGF) as produced by hMSC, could delay RGC death in a human retinal explant model of optic nerve injury. Our results showed hMSCs and the secreted growth factor PDGF-AB could substantially reduce human RGC loss and apoptosis following axotomy. The neuroprotective pathways AKT, ERK, and STAT3 were activated in the retina shortly after treatments with labeling seen in the RGC layer. A dose dependent protective effect of PDGF-AB was observed in human retinal explants but protection was not as substantial as that achieved by culturing hMSCs on the retina surface which resulted in RGC cell counts similar to those immediately post dissection. These results demonstrate that hMSCs and PDGF have strong neuroprotective action on human RGCs and may offer a translatable, therapeutic strategy to reduce degenerative visual loss. Stem Cells 2018;36:65-78.
Keywords: Degeneration; Human; Mesenchymal stem cells; Neuroprotection; Retina.
© 2017 The Authors Stem Cells published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures




References
-
- Yu S, Tanabe T, Dezawa M et al. Effects of bone marrow stromal cell injection in an experimental glaucoma model. Biochem Biophys Res Commun 2006;344:1071–1079. - PubMed
-
- Zwart I, Hill A, Al‐Allaf F et al. Umbilical cord blood mesenchymal stromal cells are neuroprotective and promote regeneration in a rat optic tract model. Exp Neurol 2009;216:439–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

