Aquaporin-1 inhibition reduces metastatic formation in a mouse model of melanoma
- PMID: 29044946
- PMCID: PMC5783831
- DOI: 10.1111/jcmm.13378
Aquaporin-1 inhibition reduces metastatic formation in a mouse model of melanoma
Abstract
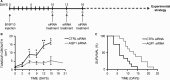
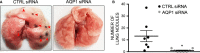
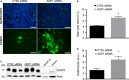
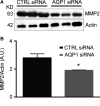
Aquaporin-1 (AQP1) is a proangiogenic water channel protein promoting endothelial cell migration. We previously reported that AQP1 silencing by RNA interference reduces angiogenesis-dependent primary tumour growth in a mouse model of melanoma. In this study, we tested the hypothesis that AQP1 inhibition also affects animal survival and lung nodule formation. Melanoma was induced by injecting B16F10 cells into the back of C57BL6J mice. Intratumoural injection of AQP1 siRNA and CTRL siRNA was performed 10 days after tumour cell implantation. Lung nodule formation was analysed after the death of the mice. Western blot was used to quantify HIF-1α, caspase-3 (CASP3) and metalloproteinase-2 (MMP2) protein levels. We found that AQP1 knock-down (KD) strongly inhibited metastatic lung nodule formation. Moreover, AQP1 siRNA-treated mice showed a twofold survival advantage compared to mice receiving CTRL siRNAs. The reduced AQP1-dependent tumour angiogenesis caused a hypoxic condition, evaluated by HIF-1α significant increase, in turn causing an increased level of apoptosis in AQP1 KD tumours, assessed by CASP3 quantification and DNA fragmentation. Importantly, a decreased level of MMP2 after AQP1 KD indicated a decreased activity against extracellular matrix associated with reduced vascularization and metastatic formation. In conclusion, these findings highlight an additional role for AQP1 as an important determinant of tumour dissemination by facilitating tumour cell extravasation and metastatic formation. This study adds knowledge on the role played by AQP1 in tumour biology and supports the view of AQP1 as a potential drug target for cancer therapy.
Keywords: antiangiogenic therapy; apoptosis; aquaporin-1; endothelial migration; extracellular matrix; melanoma; metastasis; tumour angiogenesis.
© 2017 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures


References
-
- Agre P, Sasaki S, Chrispeels MJ. Aquaporins: a family of water channel proteins. Am J Physiol. 1993; 265: F461–F461. - PubMed
-
- Agre P, Preston GM, Smith BL, et al Aquaporin CHIP: the archetypal molecular water channel. Am J Physiol. 1993; 265: F463–76. - PubMed
-
- Verkman AS. Physiological importance of aquaporin water channels. Ann Med. 2002; 34: 192–200. - PubMed
-
- Nicchia GP, Frigeri A, Liuzzi GM, et al Inhibition of aquaporin‐4 expression in astrocytes by RNAi determines alteration in cell morphology, growth, and water transport and induces changes in ischemia‐related genes. FASEB J. 2003; 17: 1508–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

