Improvements in health-related quality of life over 3 years with liraglutide 3.0 mg compared with placebo in participants with overweight or obesity
- PMID: 29045079
- PMCID: PMC5813214
- DOI: 10.1111/cob.12226
Improvements in health-related quality of life over 3 years with liraglutide 3.0 mg compared with placebo in participants with overweight or obesity
Abstract
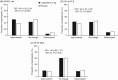
Previously in the SCALE Obesity and Prediabetes trial, at 1 year, participants with obesity (or overweight with comorbidities) and prediabetes receiving liraglutide 3.0 mg experienced greater improvements in health-related quality of life (HRQoL) than those receiving placebo. The current study extends these findings by examining 3-year changes in HRQoL. HRQoL was assessed using the obesity-specific Impact of Weight on Quality of Life-Lite (IWQOL-Lite) questionnaire, as well as the Short-Form 36 v2 (SF-36) health survey. At 3 years, mean change (±standard deviation) in IWQOL-Lite total score from baseline for liraglutide (n = 1472) was 11.0 ± 14.2, vs. 8.1 ± 14.7 for placebo (n = 738) (estimated treatment difference [ETD] 3.4 [95% confidence interval (CI): 2.0, 4.7], P < 0.0001). Mean change in SF-36 physical component summary (PCS) score from baseline for liraglutide was 3.1 ± 7.3, vs. 2.6 ± 7.6 for placebo (ETD 0.87 [95% CI: 0.17, 1.6], P = 0.0156). Mean change in SF-36 mental component summary score did not significantly differ between groups. Both IWQOL-Lite total score and PCS score demonstrated an association between greater HRQoL improvement with higher weight loss. Liraglutide 3.0 mg was also associated with improved health utility (Short-Form-6D and EuroQol-5D, mapped from IWQOL-Lite and/or SF-36) vs. placebo. Liraglutide 3.0 mg, plus diet and exercise, is associated with long-term improvements in HRQoL with obesity or overweight with comorbidity vs. placebo.
Keywords: IWQOL-Lite; SF-36 v2; liraglutide 3.0 mg; weight loss.
© 2017 The Authors. Clinical Obesity published by John Wiley & Sons Ltd on behalf of World Obesity Federation.
Figures



Similar articles
-
Improvements in health-related quality of life with liraglutide 3.0 mg compared with placebo in weight management.Clin Obes. 2016 Aug;6(4):233-42. doi: 10.1111/cob.12146. Epub 2016 May 19. Clin Obes. 2016. PMID: 27198973 Free PMC article. Clinical Trial.
-
Health-related quality of life in two randomized controlled trials of phentermine/topiramate for obesity: What mediates improvement?Qual Life Res. 2016 May;25(5):1237-44. doi: 10.1007/s11136-015-1153-x. Epub 2015 Oct 7. Qual Life Res. 2016. PMID: 26446094
-
Health-related quality of life in randomized controlled trials of lorcaserin for obesity management: what mediates improvement?Clin Obes. 2017 Dec;7(6):347-353. doi: 10.1111/cob.12207. Epub 2017 Aug 17. Clin Obes. 2017. PMID: 28815987 Clinical Trial.
-
Liraglutide: an injectable option for the management of obesity.Ann Pharmacother. 2015 Aug;49(8):938-44. doi: 10.1177/1060028015586806. Epub 2015 May 18. Ann Pharmacother. 2015. PMID: 25986009 Review.
-
Exploring the wider benefits of semaglutide treatment in obesity: insight from the STEP program.Postgrad Med. 2022 Jan;134(sup1):28-36. doi: 10.1080/00325481.2022.2150006. Postgrad Med. 2022. PMID: 36691307 Review.
Cited by
-
Liraglutide Treatment Improves Glycaemic Dysregulation, Body Composition, Cardiometabolic Variables and Uncontrolled Eating Behaviour in Adolescents with Severe Obesity.J Clin Res Pediatr Endocrinol. 2025 Mar 19;17(1):68-75. doi: 10.4274/jcrpe.galenos.2024.2023-10-10. Epub 2024 Sep 23. J Clin Res Pediatr Endocrinol. 2025. PMID: 39311553 Free PMC article.
-
Centrally Acting Agents for Obesity: Past, Present, and Future.Drugs. 2018 Jul;78(11):1113-1132. doi: 10.1007/s40265-018-0946-y. Drugs. 2018. PMID: 30014268 Free PMC article. Review.
-
The Efficacy and Safety of Liraglutide 3.0 mg for Weight Management in Obese Non-Diabetic Saudi Outpatients.Int J Gen Med. 2021 Nov 23;14:8643-8650. doi: 10.2147/IJGM.S336904. eCollection 2021. Int J Gen Med. 2021. PMID: 34849008 Free PMC article.
-
Obesity pharmacotherapy in older adults: a narrative review of evidence.Int J Obes (Lond). 2025 Mar;49(3):369-380. doi: 10.1038/s41366-024-01529-z. Epub 2024 May 6. Int J Obes (Lond). 2025. PMID: 38710803 Free PMC article. Review.
-
The Relative Value of Anti-Obesity Medications Compared to Similar Therapies.Clinicoecon Outcomes Res. 2023 Jan 26;15:51-62. doi: 10.2147/CEOR.S392276. eCollection 2023. Clinicoecon Outcomes Res. 2023. PMID: 36726966 Free PMC article.
References
-
- Ul‐Haq Z, Mackay DF, Fenwick E, Pell JP. Meta‐analysis of the association between body mass index and health‐related quality of life among adults, assessed by the SF‐36. Obesity (Silver Spring) 2013; 21: E322–E327. - PubMed
-
- Kolotkin RL, Andersen JR. A systematic review of reviews: exploring the relationship between obesity, weight loss and health‐related quality of life. Clinical Obesity 2017. https://doi.org/10.1111/cob.12203. - DOI - PMC - PubMed
-
- Kahan S, Ferguson C, David S, Devine L. Obesity drug outcome measures: results of a multi‐stakeholder critical dialogue. Curr Obes Rep 2013; 2: 128–133.
-
- The George Washington University. Obesity drug outcome measures: a consensus report of considerations regarding pharmacologic intervention. [WWW document]. Accessed 4‐11‐17.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

