Relative contributions of norspermidine synthesis and signaling pathways to the regulation of Vibrio cholerae biofilm formation
- PMID: 29045455
- PMCID: PMC5646818
- DOI: 10.1371/journal.pone.0186291
Relative contributions of norspermidine synthesis and signaling pathways to the regulation of Vibrio cholerae biofilm formation
Abstract
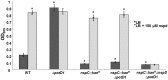
The polyamine norspermidine is one of the major polyamines synthesized by Vibrionales and has also been found in various aquatic organisms. Norspermidine is among the environmental signals that positively regulate Vibrio cholerae biofilm formation. The NspS/MbaA signaling complex detects extracellular norspermidine and mediates the response to this polyamine. Norspermidine binding to the NspS periplasmic binding protein is thought to inhibit the phosphodiesterase activity of MbaA, increasing levels of the biofilm-promoting second messenger cyclic diguanylate monophosphate, thus enhancing biofilm formation. V. cholerae can also synthesize norspermidine using the enzyme NspC as well as import it from the environment. Deletion of the nspC gene was shown to reduce accumulation of bacteria in biofilms, leading to the conclusion that intracellular norspermidine is also a positive regulator of biofilm formation. Because V. cholerae uses norspermidine to synthesize the siderophore vibriobactin it is possible that intracellular norspermidine is required to obtain sufficient amounts of iron, which is also necessary for robust biofilm formation. The objective of this study was to assess the relative contributions of intracellular and extracellular norspermidine to the regulation of biofilm formation in V. cholerae. We show the biofilm defect of norspermidine synthesis mutants does not result from an inability to produce vibriobactin as vibriobactin synthesis mutants do not have diminished biofilm forming abilities. Furthermore, our work shows that extracellular, but not intracellular norspermidine, is mainly responsible for promoting biofilm formation. We establish that the NspS/MbaA signaling complex is the dominant mediator of biofilm formation in response to extracellular norspermidine, rather than norspermidine synthesized by NspC or imported into the cell.
Conflict of interest statement
Figures





References
-
- Karatan E, Watnick P (2009) Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiology and Molecular Biology Reviews 73: 310–347. doi: 10.1128/MMBR.00041-08 - DOI - PMC - PubMed
-
- Sutherland IW (2001) The biofilm matrix–an immobilized but dynamic microbial environment. Trends in microbiology 9: 222–227. - PubMed
-
- Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical microbiology reviews 15: 167–193. doi: 10.1128/CMR.15.2.167-193.2002 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

