The Chimpanzee Model of Viral Hepatitis: Advances in Understanding the Immune Response and Treatment of Viral Hepatitis
- PMID: 29045731
- PMCID: PMC5886334
- DOI: 10.1093/ilar/ilx028
The Chimpanzee Model of Viral Hepatitis: Advances in Understanding the Immune Response and Treatment of Viral Hepatitis
Abstract
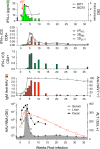
Chimpanzees (Pan troglodytes) have contributed to diverse fields of biomedical research due to their close genetic relationship to humans and in many instances due to the lack of any other animal model. This review focuses on the contributions of the chimpanzee model to research on hepatitis viruses where chimpanzees represented the only animal model (hepatitis B and C) or the most appropriate animal model (hepatitis A). Research with chimpanzees led to the development of vaccines for HAV and HBV that are used worldwide to protect hundreds of millions from these diseases and, where fully implemented, have provided immunity for entire generations. More recently, chimpanzee research was instrumental in the development of curative therapies for hepatitis C virus infections. Over a span of 40 years, this research would identify the causative agent of NonA,NonB hepatitis, validate the molecular tools for drug discovery, and provide safety and efficacy data on the therapies that now provide a rapid and complete cure of HCV chronic infections. Several cocktails of antivirals are FDA approved that eliminate the virus following 12 weeks of once-per-day oral therapy. This represents the first cure of a chronic viral disease and, once broadly implemented, will dramatically reduce the occurrence of cirrhosis and liver cancer. The recent contributions of chimpanzees to our current understanding of T cell immunity for HCV, development of novel therapeutics for HBV, and the biology of HAV are reviewed. Finally, a perspective is provided on the events leading to the cessation of the use of chimpanzees in research and the future of the chimpanzees previously used to bring about these amazing breakthroughs in human healthcare.
Keywords: HAV; HBV; HCV; antiviral; chimpanzee; hepatitis; nonhuman primate; vaccine.
© The Author 2017. Published by Oxford University Press.
Figures



References
-
- Alter HJ, Houghton M. 2000. Hepatitis C virus and eliminating post-transfusion hepatitis. Nat Med 6:1082–1086. - PubMed
-
- Alter HJ, Purcell RH, Holland PV, Popper H. 1978. Transmissible agent in non-A, non-B hepatitis. Lancet 1:459–463. - PubMed
-
- Alter HJ, Purcell RH, Shih JW, Melpolder JC, Houghton M, Choo Q-L, Kuo G. 1989. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med 321:1494–1500. - PubMed
-
- Alter HJ, Seeff LB. 2000. Recovery, persistence, and sequelae in hepatitis C virus infection: A perspective on long-term outcome. Semin Liver Dis 20:17–35. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

