GI-19007, a Novel Saccharomyces cerevisiae-Based Therapeutic Vaccine against Tuberculosis
- PMID: 29046306
- PMCID: PMC5717186
- DOI: 10.1128/CVI.00245-17
GI-19007, a Novel Saccharomyces cerevisiae-Based Therapeutic Vaccine against Tuberculosis
Abstract
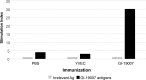
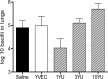
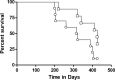
As yet, very few vaccine candidates with activity in animals against Mycobacterium tuberculosis infection have been tested as therapeutic postexposure vaccines. We recently described two pools of mycobacterial proteins with this activity, and here we describe further studies in which four of these proteins (Rv1738, Rv2032, Rv3130, and Rv3841) were generated as a fusion polypeptide and then delivered in a novel yeast-based platform (Tarmogen) which itself has immunostimulatory properties, including activation of Toll-like receptors. This platform can deliver antigens into both the class I and class II antigen presentation pathways and stimulate strong Th1 and Th17 responses. In mice this fusion vaccine, designated GI-19007, was immunogenic and elicited strong gamma interferon (IFN-γ) and interleukin-17 (IL-17) responses; despite this, they displayed minimal prophylactic activity in mice that were subsequently infected with a virulent clinical strain. In contrast, in a therapeutic model in the guinea pig, GI-19007 significantly reduced the lung bacterial load and reduced lung pathology, particularly in terms of secondary lesion development, while significantly improving survival in one-third of these animals. In further studies in which guinea pigs were vaccinated with BCG before challenge, therapeutic vaccination with GI-19007 initially improved survival versus that of animals given BCG alone, although this protective effect was gradually lost at around 400 days after challenge. Given its apparent ability to substantially limit bacterial dissemination within and from the lungs, GI-19007 potentially can be used to limit lung damage as well as facilitating chemotherapeutic regimens in infected individuals.
Keywords: BCG vaccine; T cells; Th1 cells; Th17 cells; recombinant yeast vaccine; therapeutic; therapeutic vaccine; tuberculosis.
Copyright © 2017 King et al.
Figures







Similar articles
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
Protection of mice from Mycobacterium tuberculosis by ID87/GLA-SE, a novel tuberculosis subunit vaccine candidate.Vaccine. 2011 Oct 13;29(44):7842-8. doi: 10.1016/j.vaccine.2011.07.094. Epub 2011 Aug 2. Vaccine. 2011. PMID: 21816196 Free PMC article.
-
The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M. tuberculosis-infected guinea pigs.Infect Immun. 2004 Nov;72(11):6622-32. doi: 10.1128/IAI.72.11.6622-6632.2004. Infect Immun. 2004. PMID: 15501795 Free PMC article.
-
Effects of DNA- and Mycobacterium bovis BCG-based delivery of the Flt3 ligand on protective immunity to Mycobacterium tuberculosis.Infect Immun. 2007 Nov;75(11):5368-75. doi: 10.1128/IAI.00322-07. Epub 2007 Aug 27. Infect Immun. 2007. PMID: 17724075 Free PMC article.
-
First in humans: a new molecularly defined vaccine shows excellent safety and strong induction of long-lived Mycobacterium tuberculosis-specific Th1-cell like responses.Hum Vaccin. 2010 Dec;6(12):1007-15. doi: 10.4161/hv.6.12.13143. Epub 2010 Dec 1. Hum Vaccin. 2010. PMID: 21178394 Review.
Cited by
-
The effect of antigen dose on T cell-targeting vaccine outcome.Hum Vaccin Immunother. 2019;15(2):407-411. doi: 10.1080/21645515.2018.1527496. Epub 2018 Oct 25. Hum Vaccin Immunother. 2019. PMID: 30277831 Free PMC article.
-
Sneaking Out for Happy Hour: Yeast-Based Approaches to Explore and Modulate Immune Response and Immune Evasion.Genes (Basel). 2019 Aug 31;10(9):667. doi: 10.3390/genes10090667. Genes (Basel). 2019. PMID: 31480411 Free PMC article. Review.
-
Bridging the gaps to overcome major hurdles in the development of next-generation tuberculosis vaccines.Front Immunol. 2023 Aug 11;14:1193058. doi: 10.3389/fimmu.2023.1193058. eCollection 2023. Front Immunol. 2023. PMID: 37638056 Free PMC article. Review.
-
Engineering Saccharomyces cerevisiae for medical applications.Microb Cell Fact. 2025 Jan 9;24(1):12. doi: 10.1186/s12934-024-02625-5. Microb Cell Fact. 2025. PMID: 39789534 Free PMC article. Review.
-
Development of a Potential Yeast-Based Vaccine Platform for Theileria parva Infection in Cattle.Front Immunol. 2021 Jul 8;12:674484. doi: 10.3389/fimmu.2021.674484. eCollection 2021. Front Immunol. 2021. PMID: 34305904 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

