Complement Activation in Peritoneal Dialysis-Induced Arteriolopathy
- PMID: 29046343
- PMCID: PMC5748916
- DOI: 10.1681/ASN.2017040436
Complement Activation in Peritoneal Dialysis-Induced Arteriolopathy
Abstract
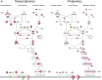
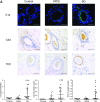
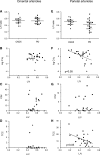
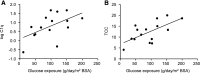
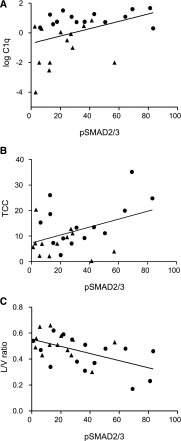
Cardiovascular disease (CVD) is the leading cause of increased mortality in patients with CKD and is further aggravated by peritoneal dialysis (PD). Children are devoid of preexisting CVD and provide unique insight into specific uremia- and PD-induced pathomechanisms of CVD. We obtained peritoneal specimens from children with stage 5 CKD at time of PD catheter insertion (CKD5 group), children with established PD (PD group), and age-matched nonuremic controls (n=6/group). We microdissected omental arterioles from tissue layers not directly exposed to PD fluid and used adjacent sections of four arterioles per patient for transcriptomic and proteomic analyses. Findings were validated in omental and parietal arterioles from independent pediatric control (n=5), CKD5 (n=15), and PD (n=15) cohorts. Transcriptomic analysis revealed differential gene expression in control versus CKD5 arterioles and in CKD5 versus PD arterioles. Gene ontology analyses revealed activation of metabolic processes in CKD5 arterioles and of inflammatory, immunologic, and stress-response cascades in PD arterioles. PD arterioles exhibited particular upregulation of the complement system and respective regulatory pathways, with concordant findings at the proteomic level. In the validation cohorts, PD specimens had the highest abundance of omental and parietal arteriolar C1q, C3d, terminal complement complex, and phosphorylated SMAD2/3, a downstream effector of TGF-β Furthermore, in the PD parietal arterioles, C1q and terminal complement complex abundance correlated with the level of dialytic glucose exposure, abundance of phosphorylated SMAD2/3, and degree of vasculopathy. We conclude that PD fluids activate arteriolar complement and TGF-β signaling, which quantitatively correlate with the severity of arteriolar vasculopathy.
Keywords: TGF-beta; arteriosclerosis; children; complement; peritoneal dialysis; vascular disease.
Copyright © 2018 by the American Society of Nephrology.
Figures




References
-
- McDonald SP, Craig JC; Australian and New Zealand Paediatric Nephrology Association : Long-term survival of children with end-stage renal disease. N Engl J Med 350: 2654–2662, 2004 - PubMed
-
- Chesnaye N, Bonthuis M, Schaefer F, Groothoff JW, Verrina E, Heaf JG, Jankauskiene A, Lukosiene V, Molchanova EA, Mota C, Peco-Antić A, Ratsch IM, Bjerre A, Roussinov DL, Sukalo A, Topaloglu R, Van Hoeck K, Zagozdzon I, Jager KJ, Van Stralen KJ; ESPN/ERA–EDTA registry : Demographics of paediatric renal replacement therapy in Europe: A report of the ESPN/ERA-EDTA registry. Pediatr Nephrol 29: 2403–2410, 2014 - PubMed
-
- Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, Mehls O, Schaefer F: Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation 106: 100–105, 2002 - PubMed
-
- de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, Collart F, Finne P, Heaf JG, De Meester J, Wetzels JF, Rosendaal FR, Dekker FW: Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 302: 1782–1789, 2009 - PubMed
-
- Kari JA, Donald AE, Vallance DT, Bruckdorfer KR, Leone A, Mullen MJ, Bunce T, Dorado B, Deanfield JE, Rees L: Physiology and biochemistry of endothelial function in children with chronic renal failure. Kidney Int 52: 468–472, 1997 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

