T Cell-Derived CD70 Delivers an Immune Checkpoint Function in Inflammatory T Cell Responses
- PMID: 29046346
- PMCID: PMC5687300
- DOI: 10.4049/jimmunol.1700380
T Cell-Derived CD70 Delivers an Immune Checkpoint Function in Inflammatory T Cell Responses
Abstract
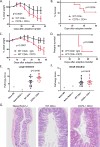
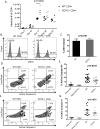
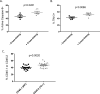
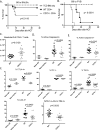
The CD27-CD70 pathway is known to provide a costimulatory signal, with CD70 expressed on APCs and CD27 functions on T cells. Although CD70 is also expressed on activated T cells, it remains unclear how T cell-derived CD70 affects T cell function. Therefore, we have assessed the role of T cell-derived CD70 using adoptive-transfer models, including autoimmune inflammatory bowel disease and allogeneic graft-versus-host disease. Surprisingly, compared with wild-type T cells, CD70-/- T cells caused more severe inflammatory bowel disease and graft-versus-host disease and produced higher levels of inflammatory cytokines. Mechanistic analyses reveal that IFN-γ induces CD70 expression in T cells, and CD70 limits T cell expansion via a regulatory T cell-independent mechanism that involves caspase-dependent T cell apoptosis and upregulation of inhibitory immune checkpoint molecules. Notably, T cell-intrinsic CD70 signaling contributes, as least in part, to the inhibitory checkpoint function. Overall, our findings demonstrate for the first time, to our knowledge, that T cell-derived CD70 plays a novel immune checkpoint role in inhibiting inflammatory T cell responses. This study suggests that T cell-derived CD70 performs a critical negative feedback function to downregulate inflammatory T cell responses.
Copyright © 2017 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no special/competing interests to disclose.
Figures



References
-
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annual review of immunology. 2005;23:23–68. - PubMed
-
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. - PubMed
-
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. The Journal of experimental medicine. 2000;192:1027–1034. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

