Excessive glucocorticoid-induced muscle MuRF1 overexpression is independent of Akt/FoXO1 pathway
- PMID: 29046370
- PMCID: PMC5691142
- DOI: 10.1042/BSR20171056
Excessive glucocorticoid-induced muscle MuRF1 overexpression is independent of Akt/FoXO1 pathway
Abstract
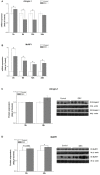
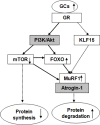
The ubiquitin-proteasome system (UPS)-dependent proteolysis plays a major role in the muscle catabolic action of glucocorticoids (GCs). Atrogin-1 and muscle-specific RING finger protein 1 (MuRF1), two E3 ubiquitin ligases, are uniquely expressed in muscle. It has been previously demonstrated that GC treatment induced MuRF1 and atrogin-1 overexpression. However, it is yet unclear whether the higher pharmacological dose of GCs induced muscle protein catabolism through MuRF1 and atrogin-1. In the present study, the role of atrogin-1 and MuRF1 in C2C12 cells protein metabolism during excessive dexamethasone (DEX) was studied. The involvement of Akt/forkhead box O1 (FoXO1) signaling pathway and the cross-talk between anabolic regulator mammalian target of rapamycin (mTOR) and catabolic regulator FoXO1 were investigated. High concentration of DEX increased MuRF1 protein level in a time-dependent fashion (P<0.05), while had no detectable effect on atrogin-1 protein (P>0.05). FoXO1/3a (Thr24/32) phosphorylation was enhanced (P<0.05), mTOR phosphorylation was suppressed (P<0.05), while Akt protein expression was not affected (P>0.05) by DEX. RU486 treatment inhibited the DEX-induced increase of FoXO1/3a phosphorylation (P<0.05) and MuRF1 protein; LY294002 (LY) did not restore the stimulative effect of DEX on the FoXO1/3a phosphorylation (P>0.05), but inhibited the activation of MuRF1 protein induced by DEX (P<0.05); rapamycin (RAPA) inhibited the stimulative effect of DEX on the FoXO1/3a phosphorylation and MuRF1 protein (P<0.05).
Keywords: FoXO1; MuRF1; glucocorticoid; muscle cell; protein metabolism.
© 2017 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures





Similar articles
-
Glycine Regulates Protein Turnover by Activating Protein Kinase B/Mammalian Target of Rapamycin and by Inhibiting MuRF1 and Atrogin-1 Gene Expression in C2C12 Myoblasts.J Nutr. 2016 Dec;146(12):2461-2467. doi: 10.3945/jn.116.231266. Epub 2016 Oct 26. J Nutr. 2016. PMID: 27798331
-
Imoxin prevents dexamethasone-induced promotion of muscle-specific E3 ubiquitin ligases and stimulates anabolic signaling in C2C12 myotubes.Biomed Pharmacother. 2020 Aug;128:110238. doi: 10.1016/j.biopha.2020.110238. Epub 2020 May 22. Biomed Pharmacother. 2020. PMID: 32450522
-
Suppression of atrogin-1 and MuRF1 prevents dexamethasone-induced atrophy of cultured myotubes.Metabolism. 2013 Oct;62(10):1495-502. doi: 10.1016/j.metabol.2013.05.018. Epub 2013 Jul 15. Metabolism. 2013. PMID: 23866982
-
Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1.Am J Physiol Endocrinol Metab. 2014 Sep 15;307(6):E469-84. doi: 10.1152/ajpendo.00204.2014. Epub 2014 Aug 5. Am J Physiol Endocrinol Metab. 2014. PMID: 25096180 Free PMC article. Review.
-
The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy.Pflugers Arch. 2011 Mar;461(3):325-35. doi: 10.1007/s00424-010-0919-9. Epub 2011 Jan 11. Pflugers Arch. 2011. PMID: 21221630 Review.
Cited by
-
Diabetic Muscular Atrophy: Molecular Mechanisms and Promising Therapies.Front Endocrinol (Lausanne). 2022 Jun 30;13:917113. doi: 10.3389/fendo.2022.917113. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35846289 Free PMC article. Review.
-
Cornflower Extract and Its Active Components Alleviate Dexamethasone-Induced Muscle Wasting by Targeting Cannabinoid Receptors and Modulating Gut Microbiota.Nutrients. 2024 Apr 11;16(8):1130. doi: 10.3390/nu16081130. Nutrients. 2024. PMID: 38674820 Free PMC article.
-
FOXO1 Is a Key Mediator of Glucocorticoid-Induced Expression of Tristetraprolin in MDA-MB-231 Breast Cancer Cells.Int J Mol Sci. 2022 Nov 8;23(22):13673. doi: 10.3390/ijms232213673. Int J Mol Sci. 2022. PMID: 36430156 Free PMC article.
-
Elephant seal muscle cells adapt to sustained glucocorticoid exposure by shifting their metabolic phenotype.Am J Physiol Regul Integr Comp Physiol. 2021 Sep 1;321(3):R413-R428. doi: 10.1152/ajpregu.00052.2021. Epub 2021 Jul 14. Am J Physiol Regul Integr Comp Physiol. 2021. PMID: 34260302 Free PMC article.
-
Irisin/FNDC5 influences myogenic markers on skeletal muscle following high and moderate-intensity exercise training in STZ-diabetic rats.3 Biotech. 2022 Sep;12(9):193. doi: 10.1007/s13205-022-03253-9. Epub 2022 Jul 26. 3 Biotech. 2022. PMID: 35910290 Free PMC article.
References
-
- Matthews D.E., (1999) Proteins and amino acids. In Modern Nutrition in Health and Diseases, 9th edn (Shils M.E., Olson J.A., Shike M. and Ross A.C., eds), pp. 11–48, Williams & Wilkins, Baltimore, MD
-
- Hoffman E.P. and Nader G.A. (2004) Balancing muscle hypertrophy and atrophy. Nat. Med. 10, 584–585 - PubMed
-
- Goldberg A.L., Tischler M., DeMartino G. and Griffin G. (1980) Hormonal regulation of protein degradation and synthesis in skeletal muscle. Fed. Proc. 39, 31–36 - PubMed
-
- Löfberg E., Gutierrez A., Wernerman J., Anderstam B., Mitch W.E., Price S.R. et al. (2002) Effects of high doses of glucocorticoids on free amino acids, ribosomes and protein turnover in human muscle. Eur. J. Clin. Invest. 32, 345–353 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

