The Hybrid Incompatibility Genes Lhr and Hmr Are Required for Sister Chromatid Detachment During Anaphase but Not for Centromere Function
- PMID: 29046402
- PMCID: PMC5714459
- DOI: 10.1534/genetics.117.300390
The Hybrid Incompatibility Genes Lhr and Hmr Are Required for Sister Chromatid Detachment During Anaphase but Not for Centromere Function
Abstract
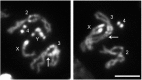
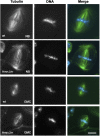
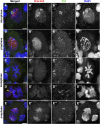
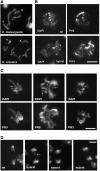
Crosses between Drosophila melanogaster females and Drosophila simulans males produce hybrid sons that die at the larval stage. This hybrid lethality is suppressed by loss-of-function mutations in the D. melanogaster Hybrid male rescue (Hmr) or in the D. simulans Lethal hybrid rescue (Lhr) genes. Previous studies have shown that Hmr and Lhr interact with heterochromatin proteins and suppress expression of transposable elements within D. melanogaster It also has been proposed that Hmr and Lhr function at the centromere. We examined mitotic divisions in larval brains from Hmr and Lhr single mutants and Hmr; Lhr double mutants in D. melanogaster In none of the mutants did we observe defects in metaphase chromosome alignment or hyperploid cells, which are hallmarks of centromere or kinetochore dysfunction. In addition, we found that Hmr-HA and Lhr-HA do not colocalize with centromeres either during interphase or mitotic division. However, all mutants displayed anaphase bridges and chromosome aberrations resulting from the breakage of these bridges, predominantly at the euchromatin-heterochromatin junction. The few dividing cells present in hybrid males showed fuzzy and irregularly condensed chromosomes with unresolved sister chromatids. Despite this defect in condensation, chromosomes in hybrids managed to align on the metaphase plate and undergo anaphase. We conclude that there is no evidence for a centromeric function of Hmr and Lhr within D. melanogaster nor for a centromere defect causing hybrid lethality. Instead, we find that Hmr and Lhr are required in D. melanogaster for detachment of sister chromatids during anaphase.
Keywords: Drosophila; Hmr; Lhr; anaphase; chromosome aberrations; interspecific hybrids; sister chromatid separation.
Copyright © 2017 by the Genetics Society of America.
Figures





Similar articles
-
The Hmr and Lhr hybrid incompatibility genes suppress a broad range of heterochromatic repeats.PLoS Genet. 2014 Mar 20;10(3):e1004240. doi: 10.1371/journal.pgen.1004240. eCollection 2014 Mar. PLoS Genet. 2014. PMID: 24651406 Free PMC article.
-
A screen for F1 hybrid male rescue reveals no major-effect hybrid lethality loci in the Drosophila melanogaster autosomal genome.G3 (Bethesda). 2014 Oct 27;4(12):2451-60. doi: 10.1534/g3.114.014076. G3 (Bethesda). 2014. PMID: 25352540 Free PMC article.
-
The Integrity of the HMR complex is necessary for centromeric binding and reproductive isolation in Drosophila.PLoS Genet. 2021 Aug 23;17(8):e1009744. doi: 10.1371/journal.pgen.1009744. eCollection 2021 Aug. PLoS Genet. 2021. PMID: 34424906 Free PMC article.
-
Frontier questions about sister chromatid separation in anaphase.Bioessays. 1995 Jun;17(6):519-26. doi: 10.1002/bies.950170608. Bioessays. 1995. PMID: 7575493 Review.
-
Modifying sister chromatid cohesion for meiosis.J Cell Sci. 2004 Aug 15;117(Pt 18):4017-23. doi: 10.1242/jcs.01352. J Cell Sci. 2004. PMID: 15316077 Review.
Cited by
-
Multi-tissue characterization of the constitutive heterochromatin proteome in Drosophila identifies a link between satellite DNA organization and transposon repression.PLoS Biol. 2025 Jan 15;23(1):e3002984. doi: 10.1371/journal.pbio.3002984. eCollection 2025 Jan. PLoS Biol. 2025. PMID: 39813297 Free PMC article.
-
Drosophila Satellite Repeats at the Intersection of Chromatin, Gene Regulation and Evolution.Prog Mol Subcell Biol. 2021;60:1-26. doi: 10.1007/978-3-030-74889-0_1. Prog Mol Subcell Biol. 2021. PMID: 34386870
-
Phenotypic characterization of diamond (dind), a Drosophila gene required for multiple aspects of cell division.Chromosoma. 2018 Dec;127(4):489-504. doi: 10.1007/s00412-018-0680-y. Epub 2018 Aug 18. Chromosoma. 2018. PMID: 30120539
-
Altered Localization of Hybrid Incompatibility Proteins in Drosophila.Mol Biol Evol. 2019 Aug 1;36(8):1783-1792. doi: 10.1093/molbev/msz105. Mol Biol Evol. 2019. PMID: 31038678 Free PMC article.
-
Regulation of Nucleolar Dominance in Drosophila melanogaster.Genetics. 2020 Apr;214(4):991-1004. doi: 10.1534/genetics.119.302471. Epub 2020 Mar 2. Genetics. 2020. PMID: 32122935 Free PMC article.
References
-
- Benna C., Bonaccorsi S., Wulbeck C., Helfrich-Forster C., Gatti M., et al. , 2010. Drosophila timeless2 is required for chromosome stability and circadian photoreception. Curr. Biol. 20: 346–352. - PubMed
-
- Bhat M. A., Philp A. V., Glover D. M., Bellen H. J., 1996. Chromatid segregation at anaphase requires the barren product, a novel chromosome-associated protein that interacts with topoisomerase II. Cell 87: 1103–1114. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

