Path perturbation detection tasks reduce MSTd neuronal self-movement heading responses
- PMID: 29046430
- PMCID: PMC5866476
- DOI: 10.1152/jn.00958.2016
Path perturbation detection tasks reduce MSTd neuronal self-movement heading responses
Abstract
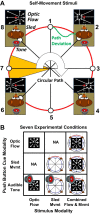
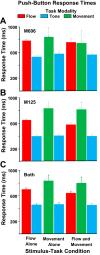
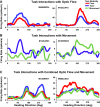
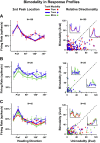
We presented optic flow and real movement heading stimuli while recording MSTd neuronal activity. Monkeys were alternately engaged in three tasks: visual detection of optic flow heading perturbations, vestibular detection of real movement heading perturbations, and auditory detection of brief tones. Push-button RTs were fastest for tones and slower for visual and vestibular heading perturbations, suggesting that the tone detection task was easier. Neuronal heading selectivity was strongest during the tone detection task, and weaker during the visual and vestibular heading perturbation detection tasks. Heading selectivity was weaker during visual and vestibular path perturbation detection, despite our presented heading cues only in the visual and vestibular modalities. We conclude that focusing on the self-movement transients of path perturbation distracted the monkeys from their heading and reduced neuronal responsiveness to heading direction. NEW & NOTEWORTHY Heading analysis is critical for steering and navigation. We recorded the activity of monkey cortical heading neurons during naturalistic self-movement. When the monkeys were required to respond to transient changes in their path, neuronal responses to heading direction were diminished. This suggests that the need to respond to momentary path perturbations reduces your ability to process your heading direction.
Keywords: attention; cortex; extrastriate; optic flow; visual motion.
Figures

Similar articles
-
Navigational path integration by cortical neurons: origins in higher-order direction selectivity.J Neurophysiol. 2015 Mar 15;113(6):1896-906. doi: 10.1152/jn.00197.2014. Epub 2015 Jan 14. J Neurophysiol. 2015. PMID: 25589586 Free PMC article.
-
Steering Transforms the Cortical Representation of Self-Movement from Direction to Destination.J Neurosci. 2015 Dec 9;35(49):16055-63. doi: 10.1523/JNEUROSCI.2368-15.2015. J Neurosci. 2015. PMID: 26658859 Free PMC article.
-
Evidence for a Causal Contribution of Macaque Vestibular, But Not Intraparietal, Cortex to Heading Perception.J Neurosci. 2016 Mar 30;36(13):3789-98. doi: 10.1523/JNEUROSCI.2485-15.2016. J Neurosci. 2016. PMID: 27030763 Free PMC article.
-
Neuronal correlates of optic flow stimulation.Ann N Y Acad Sci. 1992 May 22;656:205-19. doi: 10.1111/j.1749-6632.1992.tb25210.x. Ann N Y Acad Sci. 1992. PMID: 1599144 Review.
-
Vestibular System and Self-Motion.Front Cell Neurosci. 2018 Nov 22;12:456. doi: 10.3389/fncel.2018.00456. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30524247 Free PMC article. Review.
Cited by
-
The Effects of Depth Cues and Vestibular Translation Signals on the Rotation Tolerance of Heading Tuning in Macaque Area MSTd.eNeuro. 2020 Nov 19;7(6):ENEURO.0259-20.2020. doi: 10.1523/ENEURO.0259-20.2020. Print 2020 Nov/Dec. eNeuro. 2020. PMID: 33127626 Free PMC article.
-
Accuracy optimized neural networks do not effectively model optic flow tuning in brain area MSTd.Front Neurosci. 2024 Sep 2;18:1441285. doi: 10.3389/fnins.2024.1441285. eCollection 2024. Front Neurosci. 2024. PMID: 39286477 Free PMC article.
-
Modeling Physiological Sources of Heading Bias from Optic Flow.eNeuro. 2021 Nov 17;8(6):ENEURO.0307-21.2021. doi: 10.1523/ENEURO.0307-21.2021. Print 2021 Nov-Dec. eNeuro. 2021. PMID: 34642226 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

