Flow Cytometric Detection of PrPSc in Neurons and Glial Cells from Prion-Infected Mouse Brains
- PMID: 29046463
- PMCID: PMC5730779
- DOI: 10.1128/JVI.01457-17
Flow Cytometric Detection of PrPSc in Neurons and Glial Cells from Prion-Infected Mouse Brains
Abstract
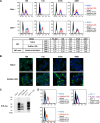
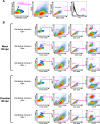
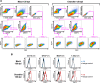
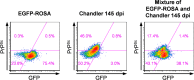
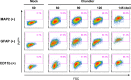
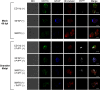
In prion diseases, an abnormal isoform of prion protein (PrPSc) accumulates in neurons, astrocytes, and microglia in the brains of animals affected by prions. Detailed analyses of PrPSc-positive neurons and glial cells are required to clarify their pathophysiological roles in the disease. Here, we report a novel method for the detection of PrPSc in neurons and glial cells from the brains of prion-infected mice by flow cytometry using PrPSc-specific staining with monoclonal antibody (MAb) 132. The combination of PrPSc staining and immunolabeling of neural cell markers clearly distinguished neurons, astrocytes, and microglia that were positive for PrPSc from those that were PrPSc negative. The flow cytometric analysis of PrPSc revealed the appearance of PrPSc-positive neurons, astrocytes, and microglia at 60 days after intracerebral prion inoculation, suggesting the presence of PrPSc in the glial cells, as well as in neurons, from an early stage of infection. Moreover, the kinetic analysis of PrPSc revealed a continuous increase in the proportion of PrPSc-positive cells for all cell types with disease progression. Finally, we applied this method to isolate neurons, astrocytes, and microglia positive for PrPSc from a prion-infected mouse brain by florescence-activated cell sorting. The method described here enables comprehensive analyses specific to PrPSc-positive neurons, astrocytes, and microglia that will contribute to the understanding of the pathophysiological roles of neurons and glial cells in PrPSc-associated pathogenesis.IMPORTANCE Although formation of PrPSc in neurons is associated closely with neurodegeneration in prion diseases, the mechanism of neurodegeneration is not understood completely. On the other hand, recent studies proposed the important roles of glial cells in PrPSc-associated pathogenesis, such as the intracerebral spread of PrPSc and clearance of PrPSc from the brain. Despite the great need for detailed analyses of PrPSc-positive neurons and glial cells, methods available for cell type-specific analysis of PrPSc have been limited thus far to microscopic observations. Here, we have established a novel high-throughput method for flow cytometric detection of PrPSc in cells with more accurate quantitative performance. By applying this method, we succeeded in isolating PrPSc-positive cells from the prion-infected mouse brains via fluorescence-activated cell sorting. This allows us to perform further detailed analysis specific to PrPSc-positive neurons and glial cells for the clarification of pathological changes in neurons and pathophysiological roles of glial cells.
Keywords: cell sorting; flow cytometry; prions.
Copyright © 2017 American Society for Microbiology.
Figures

Similar articles
-
Complement 3+-astrocytes are highly abundant in prion diseases, but their abolishment led to an accelerated disease course and early dysregulation of microglia.Acta Neuropathol Commun. 2019 May 22;7(1):83. doi: 10.1186/s40478-019-0735-1. Acta Neuropathol Commun. 2019. PMID: 31118110 Free PMC article.
-
Microglia-specific NF-κB signaling is a critical regulator of prion-induced glial inflammation and neuronal loss.PLoS Pathog. 2025 Jun 18;21(6):e1012582. doi: 10.1371/journal.ppat.1012582. eCollection 2025 Jun. PLoS Pathog. 2025. PMID: 40532025 Free PMC article.
-
In Situ Temporospatial Characterization of the Glial Response to Prion Infection.Vet Pathol. 2020 Jan;57(1):90-107. doi: 10.1177/0300985819861708. Epub 2019 Jul 22. Vet Pathol. 2020. PMID: 31331254
-
The Role of Glial Cells in Neurobiology and Prion Neuropathology.Cells. 2024 May 14;13(10):832. doi: 10.3390/cells13100832. Cells. 2024. PMID: 38786054 Free PMC article. Review.
-
Neuroinflammation, Microglia, and Cell-Association during Prion Disease.Viruses. 2019 Jan 15;11(1):65. doi: 10.3390/v11010065. Viruses. 2019. PMID: 30650564 Free PMC article. Review.
Cited by
-
Isolation of living dopaminergic neurons labeled with a fluorescent ligand of the dopamine transporter from mouse substantia nigra as a new tool for basic and applied research.Front Mol Neurosci. 2022 Dec 9;15:1020070. doi: 10.3389/fnmol.2022.1020070. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36568278 Free PMC article.
-
Complement 3+-astrocytes are highly abundant in prion diseases, but their abolishment led to an accelerated disease course and early dysregulation of microglia.Acta Neuropathol Commun. 2019 May 22;7(1):83. doi: 10.1186/s40478-019-0735-1. Acta Neuropathol Commun. 2019. PMID: 31118110 Free PMC article.
-
Phagocytic Activities of Reactive Microglia and Astrocytes Associated with Prion Diseases Are Dysregulated in Opposite Directions.Cells. 2021 Jul 8;10(7):1728. doi: 10.3390/cells10071728. Cells. 2021. PMID: 34359897 Free PMC article.
-
Glial phagocytosis for synapse and toxic proteins in neurodegenerative diseases.Mol Neurodegener. 2025 Jul 9;20(1):81. doi: 10.1186/s13024-025-00870-9. Mol Neurodegener. 2025. PMID: 40629407 Free PMC article. Review.
-
Prion protein modulation of virus-specific T cell differentiation and function during acute viral infection.Immunohorizons. 2025 Jan 23;9(1):vlae002. doi: 10.1093/immhor/vlae002. Immunohorizons. 2025. PMID: 39846843 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

